ตำรามาตรฐานยาสมุนไพรไทย
Thai Herbal Pharmacopoeia
สำนักยาและวัตถุเสพติด กรมวิทยาศาสตร์การแพทย์ กระทรวงสาธารณสุข
Bureau of Drug and Narcotic, Department of Medical Sciences, Ministry of Public Health(Tinospora crispa (L.) Hook.f. & Thomson)
(Nelumbo nucifera Gaertn.)
(Centella asiatica (L.) Urb.)
(Centella Dry Extract)
(Centella Cream)
(Mesua ferrea L.)
(Piper sarmentosum Roxb.)
(Piper sarmentosum Roxb.)
(Pterocarpus santalinus L. f.)
(Santalum album L.)
(Senna tora (L.) Roxb.)
(Senna alata (L.) Roxb.)
(Senna Alata Tea)
(Piper retrofractum Vahl)
(Myristica fragrans Houtt)
(Andrographis paniculata (Burm. f.) Nees)
(Andrographis Capsules)
(Allium ascalonicum L.)
(Ocimum tenuiflorum L.)
(Curcuma longa L.)
(Turmeric Capsules)
(Turmeric Dry Extract)
(Turmeric Dry Extract Capsules)
(Arcangelisia flava (L.) Merr.)
(Curcuma sp.)
Harrisonia perforata (Blanco) Merr.
(Aristolochia pierrei Lecomte)
(Zingiber officinale Roscoe)
(Ginger Capsules)
(Ginger Tea)
(Cassia fistula L.)
(Nardostachys jatamansi (D. Don) DC.)
(Angelica sinensis (Oliv.) Diels)
Artemisia annua L.
(Ligusticum sinense Oliv. cv. Chuanxiong)
(Neopicrorhiza scrophulariiflora Pennell)
(Atractylodes lancea (Thunb.) DC.)
(Aucklandia lappa Decne)
(Terminalia chebula Retz.)
(Angelica dahurica (Hoffm.) Benth. & Hook. f. ex Franch. & Sav. var. dahurica)
(Kaempferia parviflora Wall. ex Baker)
(Hibiscus sabdariffa L.)
(Roselle Tea)
(Allium sativum L.)
(Zingiber zerumbet (L.) Sm.)
(Wurfbainia testacea (Ridl.) Škorničk.& A. D. Poulsen)
(Cannabis sativa L.)
(Myristica fragrans Houtt)
(Dracaena cochinchinensis (Lour.) S. C. Chen)
(Ficus racemosa L.)
(Hyptis suaveolens (L.) Poit.)
Clerodendrum indicum (L.) Kuntze
(Phyllanthus emblica L.)
(Citrus hystrix DC.)
(Citrus hystrix DC.)
(Areca catechu L.)
(Momordica charantia L.)
Moringa oleifera Lam.
(Aegle marmelos (L.) Corrêa)
(Solanum trilobatum L.)
(Morus alba L.)
Gynostemma pentaphyllum(Thunb.)
Makino
(Clinacanthus nutans (Burm. f.) Lindau)
(Cissus quadrangularis L.)
(Mimusops elengi L.)
(Zingiber montanum (J. König) Link. ex A. Dietr.)
(Piper betle L.)
(Capsicum annuum L.)
(Capsicum Oleoresin)
(Capsicum Gel)
(Piper nigrum L.)
(Piper nigrum L.)
(Eurycoma longifolia Jack)
(Thunbergia laurifolia Lindl.)
(Piper wallichii (Miq.) Hand.-Mazz.)
Senna garrettiana (Craib) H. S. Irwin & Barneby
(Terminalia bellirica (Gaertn.) Roxb.)
(Terminalia chebula Retz.)
(Caesalpinia bonduc (L.) H. Roxb.)
(Tarlmounia elliptica (DC.) H. Rob., S. C. Keeley, Skvaria & R. Chan)
(Hog Creeper Vine Dry Extract Capsiles)
(Hog Creeper Vine Dry Extract)
(Brachypterum scandens (Roxb.) Miq.)
(Lepidium sativum L.)
(Nigella sativa L.)
(Cuminum cyminum L.)
(Foeniculum vulgare Mill.)
(Plantago ovata Forssk.)
(Pimpinella anisum L.)
(Carum carvi L.)
(Anethum graveolens L.)
(Trachyspermum ammi (L.) Sprague)
Albizia procera (Roxb.) Benth.
(Acorus calamus L.)
(Tiliacora triandra (Colebr.) Diels)
Cyanthillium cinereum (L.) H. Rob.
(Orthosiphon aristatus (Blume) Miq.)
Murdannia loriformis (Hassk.) R. S. Rao & Kammathy
(Capparis micracantha DC.)
(Chrysopogon zizanioides (L.) Roberty)
(Cyperus rotundus L.)
(Cannabis sativa L.)
(Syzygium aromaticum (L.) Merr. & L. M. Perry)
(Boesenbergia rotunda (L.) Mansf.)
(Acanthus ebracteatus Vahl)
(Acanthus ilicifolius L.)
(Kaempferia galanga L.)
(Curcuma comosa Roxb.)
Betula alnoides Buch.-Ham. ex D. Don
Cannabis sativa L.
Carthamus tinctorius L
Mitragyna speciosa (Korth.) Havil
Mallotus repandus (Rottler) Müll. Arg
Azadirachta indica A. Juss. var. siamensis Valeton
Azadirachta indica A. Juss. var. siamensis Valeton
Punica granatum L.
Rhinacanthus nasutus (L.) Kurz
Baliospermum solanifolium (Burm.) Suresh
Curcuma aeruginosa Roxb
Boesenbergia kingii Mood & L. M. Prince
Senegalia rugata (Lam.) Britton & Rose
Acacia concinna (Willd.) DC.
Senegalia rugata (Lam.) Britton & Rose
Acacia concinna (Willd.) DC.
Senna alexandriana Mill. var. alexandriana
Cassia acutifolia Delile, Cassia angustifolia Vahl
Butea superba Roxb. ex Willd.
[Plaso superba (Roxb. ex Willd.) Kuntze, Rudolphia superba (Roxb. ex Willd.) Poir.
Pueraria candollei Graham
ex Benth. var. mirifica (Airy Shaw & Suvat.) Niyomdham
Streblus asper Lour.
Suregada multiflora (A. Juss.) Baill. (Gelonium
multiflorum A. Juss.
Plumbago zeylanica L.
Plumbago indica L.
Biancaea sappan (L.) Tod.
Ziziphus attopensis Pierre
Streblus asper Lour.
Justicia gendarussa Burm. f.
Enhalus acoroides (L. f.) Royle
Bridelia ovata Decne.
Tamarindus indica L.
Citrus × aurantiifolia (Christm.) Swingle
Garcinia mangostana L.
Blumea balsamifera (L.) DC
Persicaria odorata (Lour.) Soják
Zingiber montanum (J. König) Link ex A. Dietr.
Mammea siamensis (Miq.) T. Anderson
Citrus maxima (Burm.) Merr.
Citrus × aurantium L. ‘Som Sa’
Punica granatum L.
Rhinacanthus nasutus (L.) Kurz
| Micro-organism |
Preparation of Test Strain |
Growth Promotion | Suitability of Counting Method in the Presence of the Product | ||
| Total Aerobic Microbial Count (TAMC) | Total Yeasts and Moulds Count (TYMC) | Total Aerobic Microbial Count (TAMC) | Total Yeasts and Moulds Count (TYMC) | ||
|
Staphylococcus
aureus such as:
ATCC 6538
DMST 8013
NCIMB 9518
C.I.P. 4.83
NBRC 13276
|
Soybean-caseins
digest agar
or Soybean-casein
digest broth
30° to 35°
18 to 24 hours
|
Soybean-casein
digest agar
or Soybean-casein
digest
broth
≤100 CFU
30° to 35°
≤3 days
|
Soybean-casein
digest agar/MPN
Soybean- casein
digest broth
≤100 CFU
30° to 35°
≤3 days
|
||
| Pseudomonas aeruginosa such as: ATCC 9027 DMST 15501 NCIMB 8626 C.I.P. 82.118 NBRC 13275 |
Soybean-casein
digest agar or Soybean-casein
digest broth
30° to 35°
18 to 24 hours
|
Soybean-casein
digest agar
or Soybean-casein
digest
broth
≤100 CFU
30° to 35°
≤3 days
|
Soybean-casein
digest agar/MPN
Soybean-casein
digest
broth
≤100 CFU
30° to 35°
≤3 days
|
||
|
Bacillus
subtilis
such as:
ATCC 6633
DMST 15896
NCIMB 8054
C.I.P. 52.62
NBRC 3134
|
Soybean-casein
digest agar
or Soybean-casein
digest broth
30° to 35°
18 to 24 hours
|
Soybean-casein
digest agar
or Soybean-casein
digest
broth
≤100 CFU
30° to 35°
≤3 days
|
Soybean-casein
digest agar /MPN
Soybean-casein
digest
broth
≤100 CFU
30° to 35°
≤3 days
|
||
Table 1 (continued)
| Micro-organism |
Preparation of Test Strain |
Growth Promotion | Suitability of Counting Method in the Presence of the Product | ||
| Total Aerobic Microbial Count (TAMC) | Total Yeasts and Moulds Count (TYMC) | Total Aerobic Microbial Count (TAMC) | Total Yeasts and Moulds Count (TYMC) | ||
|
Candida
albicans such as:
ATCC10231
DMST 5815
NCIMB3179
I.P. 48.72
NBRC 1594
|
Sabouraud
dextrose agar
or Sabouraud
dextrose broth
20° to 25°
2 to 3 days
|
Soybean-casein
digest agar
≤100 CFU
30° to 35°
≤5 days
|
Sabouraud
dextrose
agar
≤100 CFU
20° to 25°
≤5 days
|
Soybean-casein
digest
agar
≤100 CFU
30° to 35°
≤5 days
MPN: not
applicable
|
Sabouraud
dextrose
agar
≤100 CFU
20° to 25°
≤5 days
|
| Aspergillus brasiliensis such as: ATCC16404
DMST15538
IMA 149007
I.P. 1431.83
NBRC 9455
|
Sabouraud
dextrose agar
or Potato
dextrose agar
20° to 25°
5 to 7 days, or until good
sporulation is
achieved
|
Soybean-casein
digest
agar
≤100 CFU
30° to 35°
≤5 days
|
Sabouraud
dextrose
agar
≤100 CFU
20° to 25°
≤5 days
|
Soybean-casein
digest
agar
≤100 CFU
30° to 35°
≤5 days
MPN: not
applicable
|
Sabouraud
dextrose
agar
≤100 CFU
20° to 25°
≤5 days
|
| Interfering Substance | Potential Neutralizing Method |
| Glutaraldehyde, mercurials Phenolics, ethanol, aldehydes, sorbate Aldehydes Quaternary Ammonium Compounds (QACs), parahydroxy benzoates (parabens), bisbiguanides QACs, iodine, parabens Mercurials Mercurials, halogens, aldehydes EDTA (edetate) |
Sodium hydrogensulfite (sodium bisulfite) Dilution Glycine Lecithin Polysorbate Thioglycolate Thiosulfate Mg2+ or Ca2+ ions |
If no suitable neutralizing method can be found, it can be assumed that the failure to
isolate the inoculated organism is attributable to the microbicidal activity of the product.
This information serves to indicate that the product is not likely to be contaminated with
the given species of the micro-organism. However, it is possible that the product only
inhibits some of the micro-organisms specified herein, but does not inhibit others not
included amongst the test strains or for which the latter are not representative. Then,
perform the test with the highest dilution factor compatible with microbial growth and the
specific acceptance criterion.
RECOVERY OF MICRO-ORGANISMS IN THE PRESENCE OF PRODUCT For each of the micro-organisms listed, separate tests are performed. Only micro-organisms of the added test strain are counted.
Membrane filtration Use membrane filters having a nominal pore size not greater than
0.45 μm. The type of filter material is chosen such that the bacteria-retaining efficiency is not
affected by the components of the sample to be investigated. For each of the micro- organisms listed, one membrane filter is used.
Transfer a suitable amount of the sample prepared as described under Preparation of the Sample, under Inoculation and Dilution, and under Neutralization/Removal of Antimicrobial Activity (preferably representing 1 g of the sample, or less if large numbers of CFU are expected) to the membrane filter, filter immediately and rinse the membrane filter with an appropriate volume of diluent.
For the determination of total aerobic microbial count (TAMC), transfer the membrane
filter to the surface of Soybean-casein digest agar. For the determination of total combined
yeasts and moulds count (TYMC), transfer the membrane to the surface of Sabouraud dextrose agar. Incubate the plates as indicated in Table 1. Perform the counting.
Plate-count methods Perform plate-count methods at least in duplicate for each medium
and use the mean count of the result.
Pour-plate method For Petri dishes 9 cm in diameter, add to the dish 1 mL of the sample prepared as described under Preparation of the Sample, under Inoculation and Dilution, and under Neutralization/Removal of Antimicrobial Activity and 15 to 20 mL of Soybean-casein digest agar or Sabouraud dextrose agar, both media being at not more than 45°. If larger Petri dishes are used, the amount of agar medium is increased accordingly. For each of the micro-organisms listed in Table 1, at least two Petri dishes are used. Incubate the plates as indicated in Table 1. Take the arithmetic mean of the counts per medium and calculate the number of CFU in the original inoculum.
Surface-spread method For Petri dishes 9 cm in diameter, add 15 to 20 mL of Soybean-casein digest agar or Sabouraud dextrose agar at about 45° to each Petri dish and allow to solidify. If larger Petri dishes are used, the volume of the agar is increased accordingly. Dry the plates, for example in a laminar-air-flow cabinet or an incubator. For each of the micro-organisms listed in Table 1, at least two Petri dishes are used. Spread a measured volume of not less than 0.1 mL of the sample prepared as described under Preparation of the Sample, under Inoculation and Dilution, and under Neutralization/Removal of Antimicrobial Activity over the surface of the medium. Incubate and count as prescribed under Pour-plate Method.
Most probable number (MPN) method The precision and accuracy of the MPN method
is less than that of the membrane filtration method or the plate-count method. Unreliable
results are obtained particularly for the enumeration of moulds. For these reason the MPN
method is reserved for the enumeration of TAMC in situations where no other method is
available. If the use of the method is justified, proceed as follows.
Prepare a series of at least three serial ten-fold dilutions of the product as described
under Preparation of the Sample, under Inoculation and Dilution, and under Neutralization/
Removal of Antimicrobial Activity. From each level of dilution, three aliquots of 1 g or 1 mL
are used to inoculate three tubes with 9 to 10 mL of Soybean-casein digest broth. If neces- sary, a surface-active agent such as polysorbate 80 or an inactivator of antimicrobial agents may be added to the medium. Thus, if three levels of dilution are prepared, nine tubes are inoculated.
Incubate all tubes at 30° to 35° for not more than 3 days. If reading of the results is
difficult or uncertain owing to the nature of the product to be examined, subculture in the
same broth, or in Soybean-casein digest agar, for 1 to 2 days at the same temperature and use these results. Determine the most probable number of micro-organisms per g or per ml of the product to be examined from Table 3.
Results and interpretation
When verifying the suitability of the membrane filtration method or the plate-count
method, a mean count of any of the test organisms not differing by a factor greater than
2 from the value of the control defined in Inoculation and Dilution in the absence of the
product must be obtained. When verifying the suitability of the MPN method the calculated
value from the inoculum must be within 95 per cent confidence limits of the results obtained
with the control.
If the above criteria cannot be met for one or more of the organisms tested with any of the described methods, the method and test conditions that come closest to the criteria are used to test the product.
Table 3 Most Probable Number (MPN) Values of Micro-organisms
| Observed Combinations of Numbers of Tubes Showing Growth in Each Set | MPN per g or per mL of Product | 95 Per Cent Confidence Limits | ||
| Number of Grams or Millilitres of Product per Tube | ||||
| 0.1 | 0.01 | 0.001 | ||
| 0 | 0 | 0 | <3 | 0-9.4 |
| 0 | 0 | 1 | 3 | 0.1-9.50 |
| 0 | 1 | 0 | 3 | 0.1-10 |
| 0 | 1 | 1 | 6.1 | 1.2-17 |
| 0 | 2 | 0 | 6.2 | 1.2-17 |
| 0 | 3 | 0 | 9.4 | 3.5-35 |
| 1 | 0 | 0 | 3.6 | 0.2-17 |
| 1 | 0 | 1 | 7.2 | 1.2-17 |
| 1 | 0 | 2 | 11 | 4-35 |
| 1 | 1 | 0 | 7.4 | 1.3-20 |
| 1 | 1 | 1 | 11 | 4-25 |
| 1 | 2 | 0 | 11 | 4-25 |
| 1 | 2 | 1 | 15 | 5-38 |
| 1 | 3 | 0 | 16 | 5-38 |
| 2 | 0 | 0 | 9.2 | 1.5-35 |
| 2 | 0 | 1 | 14 | 4-35 |
| 2 | 0 | 2 | 20 | 5-38 |
| 2 | 1 | 0 | 15 | 4-38 |
| 2 | 1 | 1 | 20 | 5-38 |
| 2 | 1 | 2 | 27 | 9-94 |
| 2 | 2 | 0 | 21 | 5-40 |
| 2 | 2 | 1 | 28 | 9-94 |
| 2 | 2 | 2 | 35 | 9-94 |
Table 3 (Continued)
| Observed Combinations of Numbers of Tubes Showing Growth in Each Set | MPN per g or per mL of Product | 95 Per Cent Confidence Limits | ||
| Number of Grams or Millilitres of Product per Tube | ||||
| 0.1 | 0.01 | 0.001 | ||
| 2 | 3 | 0 | 29 | 9-94 |
| 2 | 3 | 1 | 36 | 9-94 |
| 3 | 0 | 0 | 23 | 5-94 |
| 3 | 0 | 1 | 38 | 9-104 |
| 3 | 0 | 2 | 64 | 16-181 |
| 3 | 1 | 0 | 43 | 9-181 |
| 3 | 1 | 1 | 75 | 17-199 |
| 3 | 1 | 2 | 120 | 30-360 |
| 3 | 1 | 3 | 160 | 30-380 |
| 3 | 2 | 0 | 93 | 18-360 |
| 3 | 2 | 1 | 150 | 30-380 |
| 3 | 2 | 2 | 210 | 30-400 |
| 3 | 2 | 3 | 290 | 90-990 |
| 3 | 3 | 0 | 240 | 40-990 |
| 3 | 3 | 1 | 460 | 90-1980 |
| 3 | 3 | 2 | 1100 | 200-4000 |
| 3 | 3 | 3 | >1100 | |
TESTING OF PRODUCTS
Amount used for the test
Unless otherwise prescribed, use 10 g or 10 mL of the product to be examined taken with the precautions referred to above. For fluids or solids in aerosol form, sample 10 containers. For transdermal patches, sample 10 patches.
The amount to be tested may be reduced for active substances that will be formulated
in the following conditions: the amount per dosage unit (e.g., tablet, capsule, injection) is
less than or equal to 1 mg or the amount per g or per mL (for preparations not presented in
dosage units) is less than 1 mg. In these cases, the amount to be tested is not less than the
amount present in 10 dosage units or 10 g or 10 mL of the product.
For materials used as active substances where sample quantity is limited or batch size is
extremely small (i.e., less than 1000 mL or 1000 g), the amount tested shall be 1 per cent of
the batch unless a lesser amount is prescribed or justified and authorized.
For products where the total number of entities in a batch is less than 200 (e.g., samples
used in clinical trials), the sample size may be reduced to 2 units, or 1 unit if the size is less than 100.
Select the sample(s) at random from the bulk material or from the available containers of
the preparation. To obtain the required quantity, mix the contents of a sufficient number of
containers to provide the sample.
Examination of the product
MEMBRANE FILTRATION Use a filtration apparatus designed to allow the transfer of the
filter to the medium. Prepare the sample using a method that has been shown suitable as
described in Growth Promotion Test, Suitability of the Counting Method and Negative
Controls. Transfer the appropriate amount to each of two membrane filters and filter
immediately. Wash each filter following the procedure shown to be suitable.
For the determination of TAMC, transfer one of the membrane filters to the surface of
Soybean-casein digest agar. For the determination of TYMC, transfer the other membrane
to the surface of Sabouraud dextrose agar. Incubate the plate of Soybean-casein digest agar
at 30° to 35° for 3 to 5 days and the plate of Sabouraud dextrose agar at 20° to 25° for 5 to 7
days. Calculate the number or CFU per g or per mL of product.
When examining transdermal patches, filter 10 per cent of the volume of the preparation
described under Preparation of the Sample separately through each of two sterile filter
membranes. Transfer one membrane to Soybean-casein digest agar for TAMC and the other
membrane to Sabouraud dextrose agar for TYMC.
PLATE-COUNT METHODS
Pour-plate method Prepare the sample using a method that has been shown to be
suitable as described in Growth Promotion Test, Suitability of the Counting Method and
Negative Controls. Prepare for each medium at least two Petri dishes for each level of
dilution. Incubate the plates of Soybean-casein digest agar at 30° to 35° for 3 to 5 days
and the plates of Sabouraud dextrose agar at 20° to 25° for 5 to 7 days. Select the plates
corresponding to a given dilution and showing the highest number of colonies less than
250 for TAMC and 50 for TYMC. Take the arithmetic mean per culture medium of the counts
and calculate the number of CFU per g or per ml of product.
Surface-spread method Prepare the sample using a method that has been shown to be
suitable as described in Growth Promotion Test, Suitability of the Counting Method and
Negative Controls. Prepare at least two Petri dishes for each medium and each level of
dilution. For incubation and calculation of the number of CFU proceed as described for
the pour-plate method.
MOST PROBABLE NUMBER METHOD
Prepare and dilute the sample using a method that has been shown to be suitable as
described in Growth Promotion Test, Suitability of the Counting Method and Negative
Controls. Incubate all tube at 30° to 35° for 3 to 5 days. Subculture if necessary, using the
procedure shown to be suitable. Record for each level of dilution the number of tubes
showing microbial growth. Determine the most probable number of micro-organisms per g
or per mL of the product to be examined from Table 3.
Interpretation of the results
The total aerobic microbial count (TAMC) is considered to be equal to the number of
CFU found using Soybean-casein digest agar; if colonies of fungi are detected on this
medium, they are counted as part of the TAMC. The total combined yeasts and mould count
(TYMC) is considered to be equal to the number of CFU found using Sabouraud dextrose
agar; if colonies of bacteria are detected on this medium, they are counted as part of the
TYMC. When the TYMC is expected to exceed the acceptance criterion due to the bacterial
growth, Sabouraud dextrose agar with antibiotics may be used. If the count is carried out by
the MPN method the calculated value is the TAMC.
The recommended solutions and media are described in Part II.
The limits prescribed in the Limits for Microbial Contamination (Appendix 10.5) are the
maximum acceptable limits.
Part II Test for Specified Micro-organisms
PROCEDURE
The preparation of the samples is carried out as described in Part I.
If the product to be examined has antimicrobial activity, this is insofar as possible removed
or neutralized as described in Part I.
If surface-active substances are used for sample preparation, their absence of toxicity for
micro-organisms and their compatibility with inactivators used must be demonstrated as
described in Part I.
GROWTH-PROMOTING AND INHIBITORY PROPERTIES OF THE MEDIA, SUITABILITY OF THE TEST AND NEGATIVE CONTROLS
The ability of the test to detect micro-organisms in the presence of the product to be tested must be established. Suitability must be confirmed if a change in testing performance, or the product, which may affect the outcome of the test is introduced.
Preparation of test strains
Use standardized stable suspensions of test strains or prepare them as stated below. Seed lot culture maintenance techniques (seed-lot systems) are used so that the viable micro-organisms used for inoculation are not more than five passages removed from the original master seed-lot.
AEROBIC MICRO-ORGANISMS Grow each of the bacterial test strains separately in Soybean-casein digest broth or on Soybean-casein digest agar at 30° to 35° for 18 to 24 hours. Grow the test strain for Candida albicans separately on Sabouraud dextrose agar or in Sabouraud dextrose broth at 20° to 25° for 2 to 3 days.
— Staphylococcus aureus such as ATCC 6538, DMST 8013, NCIMB 9518, C.I.P. 4.83 or
NBRC 13276;
— Pseudomonas aeruginosa such as ATCC 9027, DMST 15501, NCIMB 8626, C.I.P. 82.118 or NBRC 13275;
— Escherichia coli such as ATCC 8739, DMST 15537, NCIMB 8545, C.I.P. 53.126 or NBRC
3972;
— Salmonella enterica subsp. enterica serovar Typhimurium such as ATCC 14028, DMST
13311, or, as an alternative, Salmonella enterica subsp. enterica serovar Abony such as NCTC
6017, DMST 21863, C.I.P. 80.39, or NBRC 100797;
— Candida albicans such as ATCC 10231, DMST 5815, NCPF 3179, I.P. 48.72 or NBRC
1594.
Use Buffered sodium chloride-peptone solution pH 7.0 or Phosphate buffer pH 7.2 to
make test suspensions. Use the suspensions within 2 hours or within 24 hours if stored at 2°
to 8°.
ANAEROBIC MICRO-ORGANISM
— Clostridium sporogenes such as ATCC 11437 (DMST 15536, NCIMB 12343, C.I.P. 100651, NBRC 14293) or ATCC 19404 (DMST 15282, NCTC 532, C.I.P. 79.03). Grow the clostridial test strain under anaerobic conditions in Reinforced medium for clostridia at 30° to 35° for 24 to 48 hours. As an alternative to preparing and then diluting down a fresh suspension of vegetative cells of Cl. sporogenes, a stable spore suspension is used for test inoculation. The stable spore suspension may be maintained at 2° to 8° for a validated period.
Negative control
To verify testing conditions, a negative control is performed using the chosen diluent in
place of the test preparation. There must be no growth of micro-organisms. A negative
control is also performed when testing the products as described in Testing of Products.
A failed negative control requires an investigation.
Growth promotion and inhibitory properties of the media
Test each batch of ready-prepared medium and each batch of medium prepared either
from dehydrated medium or from ingredients.
Verify suitable properties of relevant media as described in Table 4.
TEST FOR GROWTH PROMOTING PROPERTIES, LIQUID MEDIA: inoculate a portion of the appropriate medium with a small number (not more than 100 CFU) of the appropriate microorganism. Incubate at the specified temperature for not more than the shortest period of time specified in the test. Clearly visible growth of the micro-organism comparable to that
previously obtained with a previously tested and approved batch of medium occurs.
TEST FOR GROWTH PROMOTING PROPERTIES, SOLID MEDIA: perform the surface spread method, inoculating each plate with a small number (not more than 100 CFU) of the
appropriate micro-organism. Incubate at the specified temperature for not more than
the shortest period of time specified in the test. Growth of the micro-organism comparable
to that previously obtained with a previously tested and approved batch of medium occurs.
TEST FOR INHIBITORY PROPERTIES, LIQUID OR SOLID MEDIA: inoculate the appropriate medium with at least 100 CFU of the appropriate micro-organism. Incubate at the specified temperature for not less than the longest period of time specified in the test. No growth of the test micro-organism occurs.
TEST FOR INDICATIVE PROPERTIES: perform the surface-spread method, inoculating each plate with a small number (not more than 100 CFU) of the appropriate micro-organism.
Incubate at the specified temperature for a period of time within the range specified in the
test. Colonies are comparable in appearance and indication reactions to those previously
obtained with a previously tested and approved batch of medium.
Suitability of the test method
For each product to be tested, perform the sample preparation as described in the following paragraph in Testing of Products. Add each test strain at the time of mixing, in the prescribed growth medium. Inoculate the test strains individually. Use a number of micro-organisms equivalent to not more than 100 CFU in the inoculated test preparation. Perform the test as described in the following paragraph in Testing of Products using the shortest incubation period prescribed.
The specified micro-organisms must be detected with the indication reactions as described in Testing of Products.
Any antimicrobial activity of the sample necessitates a modification of the test procedure
described in Neutralization/Removal of Antimicrobial Activity under Part I.
If for a given product the antimicrobial activity with respect to a micro-organism for
which testing is prescribed cannot be neutralized, then it is to be assumed that the inhibited
micro-organism will not be present in the product.
Table 4 Growth Promoting, Inhibitory and Indicative Properties of Media
| Test | Medium | Property | Test Strains |
| Test for bile-tolerant gram-negative bacteria | Endobacteria enrichment broth-Mossel | Growth promoting Inhibitory | E. coli P. aeruginosa S. aureus |
| Violet red bile dextrose agar | Growth promoting + indicative | E. coli P. aeruginosa |
|
| Test for Escherichia coli | MacConkey broth | Growth promoting Inhibitory | E. coli S. aureus |
| MacConkey agar | Growth promoting + indicative | E. coli | |
| Test for Pseudomonas aeruginosa | Cetrimide agar Pseudomonas for detection fluorescin Pseudomonas for detection pyocyanin |
Growth promoting Inhibitory | P. aeruginosa E. coli |
| Test for Salmonella |
Rappaport-Vassilia-dis broth Tetrathionate bile brilliant green broth |
Growth promoting Inhibitory | Salmonella enterica subsp. enterica serovar Typhimurium or Salmonella enterica subsp. enterica serovar Abony S. aureus |
| Xylose-lysine-deoxycholate agar Brilliant green agar Bismuth sulfite agar |
Growth promoting + indicative | Salmonella enterica subsp. enterica serovar Typhimurium or Salmonella enterica subsp. enterica serovar Abony |
Table 4 (Continued)
| Test | Medium | Property | Test Strains |
| Test for Staphylococcus aureus | Mannitol salt agar Baird-Parker agar Vogel-Johnson agar |
Growth promoting + indicative Inhibitory |
S. aureus E. coli |
| Test for Clostridium spp. | Reinforced medium for Clostidia | Growth promoting | Cl. sporogenes |
| Columbia agar Defibrinated sheep blood agar | Growth promoting | Cl. sporogenes | |
| Test for Candida albicans | Sabouraud dextrose broth | Growth promoting | C. albicans |
| Sabouraud dextrose agar | Growth promoting + indicative | C. albicans |
TESTING OF PRODUCTS
Bile-tolerant gram-negative bacteria
SAMPLE PREPARATION AND PRE-INCUBATION Prepare a sample using a 1 in 10 dilution of not less than 1 g of the product to be examined as described in Part I, but using Soybean-casein digest broth as the chosen diluent, mix and incubate at 20° to 25° for a time sufficient to resuscitate the bacteria but not sufficient to encourage multiplication of the organisms (usually 2 hours but not more than 5 hours).
TEST FOR ABSENCE Use the volume corresponding to 1 g of the product, as prepared in Sample Preparation and Pre-incubation, to inoculate Enterobacteria enrichment broth-
Mossel. Incubate at 30° to 35° for 24 to 48 hours. Subculture on plates of Violet red bile
dextrose agar. Incubate at 30° to 35° for 18 to 24 hours. The product passes the test if there is no growth of colonies of Gram-negative bacteria on any plate.
SEMI-QUANTITATIVE TEST
Suitability of the test method Use a number of micro-organisms equivalent to not more
than 100 CFU per g or per mL of product. Perform the test as described in the following
paragraph in Testing of Products using the shortest incubation period prescribed. The
dilution corresponding to 0.1 g or 0.1 mL of product must be positive.
Sample preparation and pre-incubation Prepare a sample using a 1 in 10 dilution of not
less than 1 g of the product to be examined as described in Part I, but using Soybean-casein
digest broth as the chosen diluent, mix and incubate at 20° to 25° for a time sufficient to
resuscitate the bacteria but not sufficient to encourage multiplication of the organisms
(usually 2 hours but not more than 5 hours).
Selection and subculture Inoculate suitable quantities of Enterobacteria enrichment
broth-Mossel with the preparation as described under Sample Preparation and Pre-incubation and/or dilutions of it containing successively 0.1 g (or 0.1 mL), 0.01 g (or 0.01 mL), and 0.001 g (or 0.001 mL) of the sample to be examined. Incubate at 30° to 35° for 24 to 48 hours. Subculture each of the cultures on a plate of Violet red bile dextrose agar to obtain selective isolation. Incubate at 30° to 35° for 18 to 24 hours.
Interpretation Growth of colonies constitutes a positive result. Note the smallest quantity
of the product that gives a positive result and the largest quantity that gives a negative result.
Determine from Table 5 the probable number of bacteria.
Table 5 Probable Number of Bacteria
| Results for Each Quantity of Product | Probable Number of Bacteria per g or per mL of Poduct |
||
| 0.1 g (or 0.1 mL) |
0.01 g (or 0.01 mL) |
0.001 g (or 0.001 mL) |
|
| + | + | + | More than 103 |
| + | + | - | Less than 103 and more than 102 |
| + | - | - | Less than 102 and more than 10 |
| - | - | - | Less than 10 |
Escherichia coli
SAMPLE PREPARATION AND PRE-INCUBATION Prepare a sample using a 1 in 10 dilution of not less than 1 g of the product to be examined as described in Part I, and use 10 mL or the portion corresponding to 1 g or 1 mL to inoculate a suitable amount (determined as
described under Suitability of the test method) of Soybean-casein digest broth, mix and
incubate at 30° to 35° for 18 to 24 hours.
SELECTION AND SUBCULTURE Transfer 1 mL of the enrichment culture to 100 mL of
MacConkey broth and incubate at 42° to 44° for 24 to 48 hours. Subculture on plates of
MacConkey agar and incubate at 30° to 35° for 18 to 72 hours.
INTERPRETATION Upon examination, if none of the colonies conforms to the description given in Table 6, the product meets the requirements of the test for absence of Escherichia coli. If colonies matching the description in Table 6 are found, proceed with further identification
IDENTIFICATION Transfer the suspect colonies individually, making subculture the
suspect colonies individually on plates of Levine eosin-methylene blue agar, and incubate at
30° to 35° for 18 to 24 hours.
Upon examination, if none of the colonies exhibits both a characteristic metallic sheen
under reflected light and a blue-black appearance under transmitted light, the product meets
the requirements of the test for absence of Escherichia coli. The presence of Escherichia coli may be confirmed by suitable cultural and, if necessary, biochemical tests. Further serological test may be performed.
Table 6 Morphology Characteristics of Escherichia coli on MacConkey Agar
| Gram Stain | Characteristic Colonial Morphology |
| Negative rods (cocco-bacilli) | Brick-red; may have surrounding zone of precipitated bile |
SEMI-QUANTITATIVE TEST
Suitability of the test method Use a number of micro-organisms equivalent to not more
than 100 CFU per g or per mL of product. Perform the test as described in the following
paragraph in Testing of Products using the shortest incubation period prescribed. The dilution
corresponding to 0.1 g or 0.1 mL of product must be positive.
Sample preparation and pre-incubation Use suitable quantities of Soybean-casein digest
broth with the preparation as described under Sample Preparation and Pre-incubation
and/or dilutions of it containing sucessively 0.1 g (or 0.1 mL), 0.01 g (or 0.01 mL), and 0.001 g (or 0.001 mL) to inoculate a suitable amount (determined as described in Suitability of the
test method) of Soybean-casein digest broth, mix and incubate at 30° to 35° for 18 to 24 hours of the sample to be examined.
Selection and subculture Shake the container, transfer 1 mL of the enrichment culture to
100 mL of MacConkey broth and incubate at 42° to 44° for 24 to 48 hours. Subculture on
plates of MacConkey agar and incubate at 30° to 35° for 18 to 72 hours.
Interpretation Growth of colonies indicates the possible presence of Escherichia coli. This is confirmed by the test for the identification of Escherichia coil. Note the smallest quantity of the product that gives a positive result and the largest quantity that gives a negative result. Determine from Table 5 the probable number of bacteria.
Salmonella species
SAMPLE PREPARATION AND PRE-INCUBATION Prepare the product to be examined as described in Part I, using the portion corresponding to not less than 10 g or 10 mL for pharmaceutical preparations and 25 g or 25 mL for herbal drug preparations. Inoculate a suitable amount (determined as described under Suitability of the Test Method) of Soybean-casein digest broth. Mix and incubate at 30° to 35° for 18 to 24 hours.
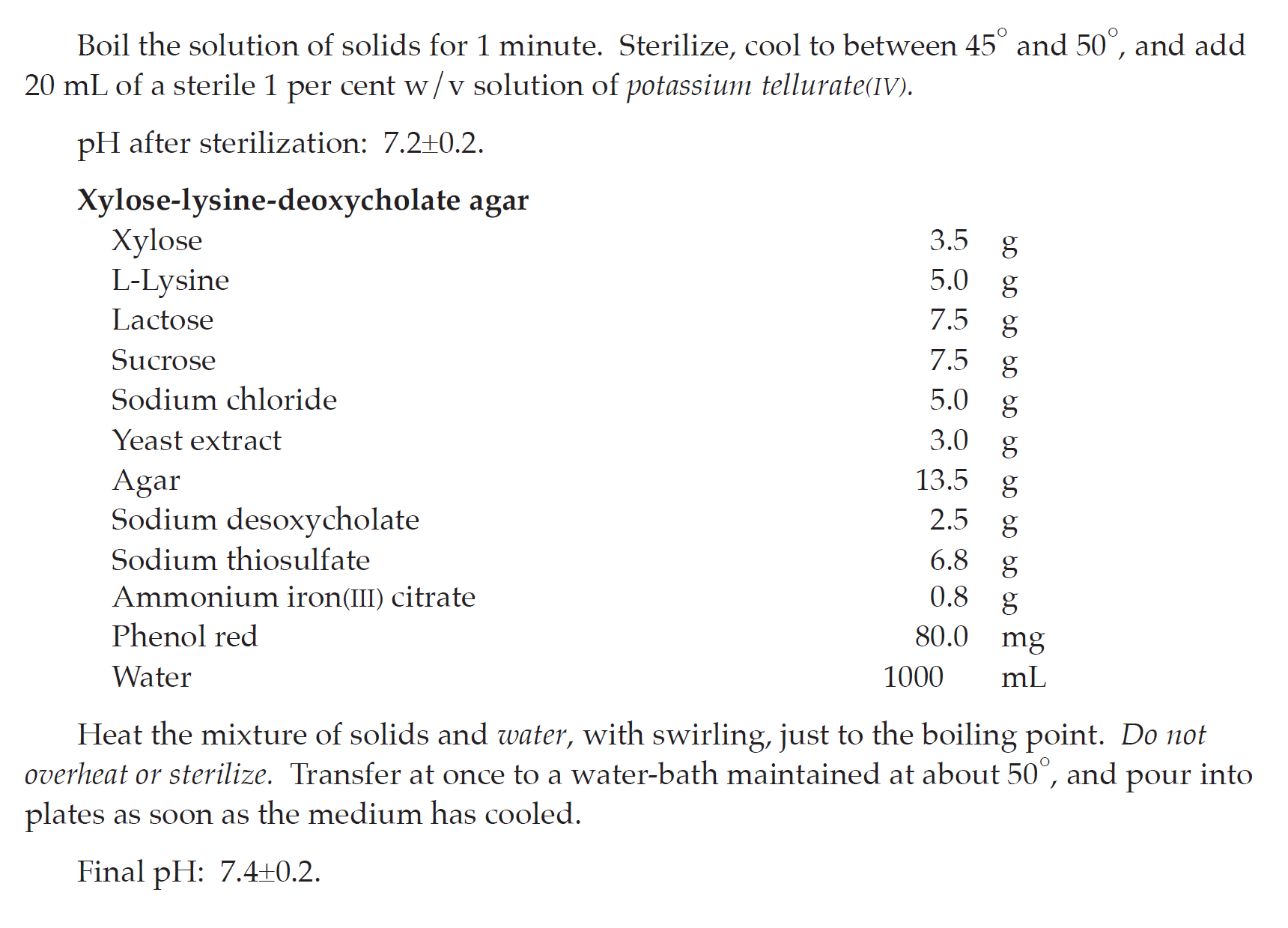
SELECTION AND SUBCULTURE Separately transfer 0.1 mL and 1 mL of the enrichment culture to 10 mL of Rappaport-Vassiliadis broth and Tetrathionate bile brilliant green broth, respectively, mix and incubate at 30° to 35° for 18 to 24 hours. Subculture on plates of Xylose-lysine-deoxycholate agar, Brilliant green agar, and Bismuth sulfite agar. Cover and invert the dishes, and incubate at 30° to 35° for 18 to 48 hours.
INTERPRETATION Upon examination, if none of the colonies conforms to the description given in Table 7, the product meets the requirements of the test for absence of the genus Salmonella. If colonies of Gram-negative rods matching the description in Table 7 are found, proceed with further identification.
IDENTIFICATION Transfer representative suspect colonies individually, by means of an
inoculating wire, to a butt-slant tube of Triple sugar-iron agar by first streaking the surface of
the slant and then stabbing the wire well beneath the surface, and incubate. If the examination discloses no evidence of tubes having alkaline (red) slants and acid (yellow) butts (with or without concomitant blackening of the butt from hydrogen sulfide production), the product meets the requirements of the test for absence of the genus Salmonella. The presence of Salmonella may be confirmed by other suitable cultural or biochemical and serological tests, if necessary.
Table 7 Morphology Characteristics of Salmonella Species on Selective Agar Media
| Selective Medium | Characteristic Colonial Morphology |
| Xylose-lysine-deoxycholate agar | Red, with or without black centres |
| Brilliant green agar | Small, transparent, colourless or pink to white opaque (frequently surrounded by pink to red zone) |
| Bismuth sulfite agar | Black or green |
Staphylococcus aureus and Pseudomonas aeruginosa
SAMPLE PREPARATION AND PRE-INCUBATION Prepare a sample using a 1 in 10 dilution of not less than 1 g of the product to be examined as described in Part I, and use 10 mL or the portion corresponding to 1 g or 1 mL to inoculate a suitable amount (determined as described under Suitability of the Tes t Method) of Soybean-casein digest broth and mix. When testing transdermal patches, filter the volume of sample corresponding to one patch of the preparation described under Preparation of the Sample in Part I through a s terile filter membrane and place in 100 mL of Soybean-casein diges t broth. Incubate at 30° to 35° for 18 to 24 hours.
SELECTION AND SUBCULTURE If growth is present, use an inoculating loop to s treak
a portion of the culture medium on the surface of Mannitol-salt agar, or Baird-Parker agar,
or Vogel-Johnson agar and of Cetrimide agar, and incubate at 30° to 35° for 18 to 72 hours.
INTERPRETATION Upon examination, if none of the plates contains colonies having
the characteris tics lis ted in Tables 8 and 9 for the media used, the product meets the
requirements for the absence of Staphylococcus aureus and Pseudomonas aeruginosa. If colonies matching the description in Table 8 and 9 are found, proceed with further identification.
IDENTIFICATION
Coagulase test (for Staphylococcus aureus) With the aid of an inoculating loop, transfer
representative suspect colonies from the agar surfaces of the Mannitol-salt agar (or
Baird-Parker agar or Vogel-Johnson agar) to individual tubes, each containing 0.5 mL of
mammalian, preferably rabbit or horse, plasma with or without suitable additives. Incubate
in a water-bath at 37°, examining the tubes at 3 hours and subsequently at suitable intervals
up to 24 hours. Test positive and negative controls simultaneously with the unknown
products. If no coagulation in any degree is observed, the product meets the requirements
of the test for absence of Staphylococcus aureus.
Oxidase and pigment tests (for Pseudomonas aeruginosa) With the aid of an inoculating
loop, s treak representative suspect colonies from the agar surfaces of Cetrimide agar on the
agar surface of Pseudomonas agar for detection of fluorescin and Pseudomonas agar for
detection of pyocyanin contained in Petri dishes. Cover and invert the inoculated media,
and incubate at 30° to 35° for not less than 3 days. Examine the s treaked surfaces under UV
light. Examine the plates to determine whether colonies having the characteris tics lis ted in
Table 9 are present.
Table 8 Morphology Characteristics of Staphylococcus aureus on Selective Agar Media
| Selective Medium | Characteristic Colonial Morphology | Gram Stain |
| Mannitol-salt agar | Yellow colonies surrounded by yellow zone | Positive cocci (in clusters) |
| Baird-Parker agar | Black, shiny colonies surrounded by clear zones of 2 to 5 mm |
Positive cocci (in clusters) |
| Vogel-Johnson agar | Black surrounded by yellow zones | Positive cocci (in clusters) |
Table 9 Morphology and Diagnos tic Characteristics of Pseudomonas aeruginosa on
Selective Agar Media
| Selective Medium |
Characteristic Colonial Morphology |
Fluorescence in UV Light |
Oxidase Test | Gram Stain |
| Cetrimide agar | Generally greenish | Greenish | Positive | Negative rods |
| Pseudomonas agar for to detection of fluorescin |
Generally colourless yellowish |
Yellowish | Positive | Negative rods |
| Pseudomonas agar for detection of pyocyanin |
Generally greenish | Blue | Positive | Negative rods |
Confirm any suspect colonial growth on one or more of the media as Pseudomonas
aeruginosa by means of the oxidase test. Upon the colonial growth, place or transfer
colonies to s trips or discs of filter paper that previously has been impregnated with
N,N-dimethyl-p-phenylenediamine dihydrochloride. If there is no development of a pink
colour, changing to purple, the product meets the requirements of the test for the absence
of Pseudomonas aeruginosa. The presence of Pseudomonas aeruginosa may be confirmed by suitable cultural and, if necessary, biochemical tests.
Candida albicans
SAMPLE PREPARATION AND PRE-INCUBATION Prepare the product to be examined as described under Preparation of the Sample and use 10 mL or the portion corresponding to
1 g or 1 mL to inoculate a suitable amount (determined as described under Suitability of the
Test Method) of Sabouraud dextrose broth and mix. Incubate at 30°to 35° for 3 to 5 days.
SELECTION AND SUBCULTURE Subculture on a plate of Sabouraud dextrose agar and
incubate at 30° to 35° for 24 to 48 hours.
INTERPRETATION When growth of white colonies may indicate the presence of Candida albicans occurs, proceed with further identification.
IDENTIFICATION Transfer the suspect colonies individually, making subculture the
suspect colonies individually on plates of a suitable selective medium1.
Upon examination, the product passes the tes t if there is no growth of colonies of Candida albicans on any plate.
Clostridium spp.
SAMPLE PREPARATION AND HEAT TREATMENT Prepare the product to be examined as described under Preparation of the Sample in Part I. Use two 10-mL portions each
corresponding to 1 g or 1 mL of the product to be examined to inoculate a suitable amount
(determined as described under Suitability of the Tes t Method) of Reinforced medium for
clos tridia. Heat one portion at 80° for 10 minutes and cool rapidly. Do not heat the other
portion. Incubate both containers under anaerobic conditions at 30° to 35° for 48 hours.
SELECTION AND SUBCULTURE After incubation, make subcultures from each container on plates of Columbia agar to which gentamicin has been added and incubate under anaerobic conditions at 30° to 35° for 48 to 72 hours.
INTERPRETATION If no growth occurs, the product passes the tes t for absence of
Clostridium spp. When growth of rods (with or without endospores) giving a negative
catalase reaction occurs, subculture each dis tinct colony from on plates of Columbia agar,
without gentamicin, and incubate at 30° to 35° for 48 to 72 hours, one plate anaerobically and
the other aerobically, to check that the organism will not grow under aerobic condition.
Examine the appearance of only anaerobic growth of Gram-positive bacilli giving
a negative catalase reaction together with the extent of hemolysis, by making subculture on
a plate of Defibrinated sheep blood agar, and also examine microscopically for spore
formation, using Gram s tain or spore s tain technique and confirmed by further suitable
biochemical and biological tes ts. The description in Table 10 gives the characteris tics of
some Clostridium species on Defibrinated sheep blood agar.
1Biggy agar, CHROMagar Candida, or Candida isolation agar is recommended.
Table 10 Characteris tics of Clostridium Species on Defibrinated Sheep Blood Agar
| Selective Species |
Colonies | Hemolysis | Spore (Staining) |
| Clostridium botulinum |
Irregular, translucent with a granular surface and indefinited fimbriated spreading edge. |
+ | Oval, central, subterminal distend bacilli |
| Clostridium perfringens |
Large, circular, convex, semitranslucent, smooth with an entire edge. |
Double zone | Oval and subterminal (very rare) |
| Clostridium tetani |
Transparent with long feathery spreading projections. |
+ | Spherical and terminal (drumstick) |
Buffer Solution and Media
Culture media may be prepared as follows, or dehydrated culture media may be used if
they have similar or comparable nutritive and selective properties for the micro-organisms
to be tes ted for.
In preparing the media according to the formulae set forth herein, dissolve the soluble
solids in the water, using heat, if necessary, to effect complete solution, and add other
ingredients. Add, if necessary, a solution of hydrochloric acid or sodium hydroxide in
quantities sufficient to yield the desired pH in the medium when it is ready for use.
Determine the pH at 25°±2°.
Where agar is called for in a formula, use agar that has a mois ture content of not more
than 15 per cent.
Unless otherwise indicated, the buffer solution and media should be dispensed and
sterilized by heating in an autoclave at 121°±2° for not less than 15 minutes, depending on
the volume to be s terilized. Store under refrigeration.
BUFFER SOLUTION
Stock buffer solution
Place 34 g of potassium dihydrogenphosphate in a 1000-mL volumetric flask, dissolve in
500 mL of water, adjus t to pH 7.2±0.2 with sodium hydroxide, dilute to 1000.0 mL with
water and mix. Dispense into containers and sterilize. Store at 2° to 8°.
Phosphate buffer pH 7.2
Prepare a mixture of 1 volume of stock buffer solution and 800 volumes of water and sterilize.
Buffered sodium chloride-peptone solution pH 7.0
| Potassium dihydrogenphosphate | 3.56 g |
| Disodium hydrogenphosphate heptahydrate | 10.89 g |
| Sodium chloride | 4.30 g |
| Peptone, dried | 1.0 g |
| Water | 1000 mL |
Polysorbate 20 or 80 may be added to obtain a 0.1 to 1.0 per cent w/v solution.
pH after sterilization: 7.0±0.1.
MEDIA
Baird-Parker agar
| Pancreatic digest of caseins | 10.0 g |
| Beef extract | 5.0 g |
| Yeast extract | 1.0 g |
| Lithium chloride | 5.0 g |
| Agar | 20.0 g |
| Glycine | 12.0 g |
| Sodium pyruvate | 10.0 g |
| Water | 950 mL |
Heat with frequent agitation, and boil for 1 minute. Sterilize, cool to between 45° and
50°, and add 10 mL of a sterile, 1 per cent w/v solution of potassium tellurate(IV) and 50 mL of egg-yolk emulsion. Mix intimately but gently, and pour into plates.
pH after s terilization: 6.8±0.2.
Preparation of the egg-yolk emulsion: Disinfect the surface of whole shell eggs,
aseptically crack the eggs, and separate out intact yolks into a s terile graduated cylinder.
Add saline TS to obtain a 3 to 7 ratio of egg-yolk to saline. Add to a sterile blender cup, and
mix at high speed for 5 seconds.
Bismuth sulfite agar
| Beef extract | 5.0 g |
| Pancreatic digest of casein | 5.0 g |
| Peptic digest of animal tissue | 5.0 g |
| Dextrose monohydrate | 5.0 g |
| Disodium hydrogenphosphate heptahydrate | 4.0 g |
| Iron(II) sulfate | 0.3 g |
| Bismuth sulfite indicator | 8.0 g |
| Agar | 20.0 g |
| Brilliant green | 25.0 mg |
| Water | 1000 mL |
Heat the mixture of solids and water, with swirling, jus t to the boiling point. Do not
overheat or sterilize. Transfer at once to a water-bath maintained at about 50°, and pour into
plates as soon as the medium has cooled.
Final pH: 7.6±0.2.
Brilliant green agar
| Yeast extract | 3.0 g |
| Peptic digest of animal tissue | 5.0 g |
| Pancreatic digest of casein | 5.0 g |
| Lactose | 10.0 g |
| Sodium chloride | 5.0 g |
| Sucrose | 10.0 g |
| Phenol red | 80.0 mg |
| Agar | 20.0 g |
| Brilliant green | 12.5 mg |
| Water | 1000 mL |
Boil the solution of solids for 1 minute. Sterilize jus t prior to use. Melt the medium,
pour into Petri dishes, and allow to cool.
pH after sterilization: 6.9±0.2.