ตำรามาตรฐานยาสมุนไพรไทย
Thai Herbal Pharmacopoeia
สำนักยาและวัตถุเสพติด กรมวิทยาศาสตร์การแพทย์ กระทรวงสาธารณสุข
Bureau of Drug and Narcotic, Department of Medical Sciences, Ministry of Public Health(Tinospora crispa (L.) Hook.f. & Thomson)
(Nelumbo nucifera Gaertn.)
(Centella asiatica (L.) Urb.)
(Centella Dry Extract)
(Centella Cream)
(Mesua ferrea L.)
(Piper sarmentosum Roxb.)
(Piper sarmentosum Roxb.)
(Pterocarpus santalinus L. f.)
(Santalum album L.)
(Senna tora (L.) Roxb.)
(Senna alata (L.) Roxb.)
(Senna Alata Tea)
(Piper retrofractum Vahl)
(Myristica fragrans Houtt)
(Andrographis paniculata (Burm. f.) Nees)
(Andrographis Capsules)
(Allium ascalonicum L.)
(Ocimum tenuiflorum L.)
(Curcuma longa L.)
(Turmeric Capsules)
(Turmeric Dry Extract)
(Turmeric Dry Extract Capsules)
(Arcangelisia flava (L.) Merr.)
(Curcuma sp.)
Harrisonia perforata (Blanco) Merr.
(Aristolochia pierrei Lecomte)
(Zingiber officinale Roscoe)
(Ginger Capsules)
(Ginger Tea)
(Cassia fistula L.)
(Nardostachys jatamansi (D. Don) DC.)
(Angelica sinensis (Oliv.) Diels)
Artemisia annua L.
(Ligusticum sinense Oliv. cv. Chuanxiong)
(Neopicrorhiza scrophulariiflora Pennell)
(Atractylodes lancea (Thunb.) DC.)
(Aucklandia lappa Decne)
(Terminalia chebula Retz.)
(Angelica dahurica (Hoffm.) Benth. & Hook. f. ex Franch. & Sav. var. dahurica)
(Kaempferia parviflora Wall. ex Baker)
(Hibiscus sabdariffa L.)
(Roselle Tea)
(Allium sativum L.)
(Zingiber zerumbet (L.) Sm.)
(Wurfbainia testacea (Ridl.) Škorničk.& A. D. Poulsen)
(Cannabis sativa L.)
(Myristica fragrans Houtt)
(Dracaena cochinchinensis (Lour.) S. C. Chen)
(Ficus racemosa L.)
(Hyptis suaveolens (L.) Poit.)
Clerodendrum indicum (L.) Kuntze
(Phyllanthus emblica L.)
(Citrus hystrix DC.)
(Citrus hystrix DC.)
(Areca catechu L.)
(Momordica charantia L.)
Moringa oleifera Lam.
(Aegle marmelos (L.) Corrêa)
(Solanum trilobatum L.)
(Morus alba L.)
Gynostemma pentaphyllum(Thunb.)
Makino
(Clinacanthus nutans (Burm. f.) Lindau)
(Cissus quadrangularis L.)
(Mimusops elengi L.)
(Zingiber montanum (J. König) Link. ex A. Dietr.)
(Piper betle L.)
(Capsicum annuum L.)
(Capsicum Oleoresin)
(Capsicum Gel)
(Piper nigrum L.)
(Piper nigrum L.)
(Eurycoma longifolia Jack)
(Thunbergia laurifolia Lindl.)
(Piper wallichii (Miq.) Hand.-Mazz.)
Senna garrettiana (Craib) H. S. Irwin & Barneby
(Terminalia bellirica (Gaertn.) Roxb.)
(Terminalia chebula Retz.)
(Caesalpinia bonduc (L.) H. Roxb.)
(Tarlmounia elliptica (DC.) H. Rob., S. C. Keeley, Skvaria & R. Chan)
(Hog Creeper Vine Dry Extract Capsiles)
(Hog Creeper Vine Dry Extract)
(Brachypterum scandens (Roxb.) Miq.)
(Lepidium sativum L.)
(Nigella sativa L.)
(Cuminum cyminum L.)
(Foeniculum vulgare Mill.)
(Plantago ovata Forssk.)
(Pimpinella anisum L.)
(Carum carvi L.)
(Anethum graveolens L.)
(Trachyspermum ammi (L.) Sprague)
Albizia procera (Roxb.) Benth.
(Acorus calamus L.)
(Tiliacora triandra (Colebr.) Diels)
Cyanthillium cinereum (L.) H. Rob.
(Orthosiphon aristatus (Blume) Miq.)
Murdannia loriformis (Hassk.) R. S. Rao & Kammathy
(Capparis micracantha DC.)
(Chrysopogon zizanioides (L.) Roberty)
(Cyperus rotundus L.)
(Cannabis sativa L.)
(Syzygium aromaticum (L.) Merr. & L. M. Perry)
(Boesenbergia rotunda (L.) Mansf.)
(Acanthus ebracteatus Vahl)
(Acanthus ilicifolius L.)
(Kaempferia galanga L.)
(Curcuma comosa Roxb.)
Betula alnoides Buch.-Ham. ex D. Don
Cannabis sativa L.
Carthamus tinctorius L
Mitragyna speciosa (Korth.) Havil
Mallotus repandus (Rottler) Müll. Arg
Azadirachta indica A. Juss. var. siamensis Valeton
Azadirachta indica A. Juss. var. siamensis Valeton
Punica granatum L.
Rhinacanthus nasutus (L.) Kurz
Baliospermum solanifolium (Burm.) Suresh
Curcuma aeruginosa Roxb
Boesenbergia kingii Mood & L. M. Prince
Senegalia rugata (Lam.) Britton & Rose
Acacia concinna (Willd.) DC.
Senegalia rugata (Lam.) Britton & Rose
Acacia concinna (Willd.) DC.
Senna alexandriana Mill. var. alexandriana
Cassia acutifolia Delile, Cassia angustifolia Vahl
Butea superba Roxb. ex Willd.
[Plaso superba (Roxb. ex Willd.) Kuntze, Rudolphia superba (Roxb. ex Willd.) Poir.
Pueraria candollei Graham
ex Benth. var. mirifica (Airy Shaw & Suvat.) Niyomdham
Streblus asper Lour.
Suregada multiflora (A. Juss.) Baill. (Gelonium
multiflorum A. Juss.
Plumbago zeylanica L.
Plumbago indica L.
Biancaea sappan (L.) Tod.
Ziziphus attopensis Pierre
Streblus asper Lour.
Justicia gendarussa Burm. f.
Enhalus acoroides (L. f.) Royle
Bridelia ovata Decne.
Tamarindus indica L.
Citrus × aurantiifolia (Christm.) Swingle
Garcinia mangostana L.
Blumea balsamifera (L.) DC
Persicaria odorata (Lour.) Soják
Zingiber montanum (J. König) Link ex A. Dietr.
Mammea siamensis (Miq.) T. Anderson
Citrus maxima (Burm.) Merr.
Citrus × aurantium L. ‘Som Sa’
Punica granatum L.
Rhinacanthus nasutus (L.) Kurz
Baliospermum Solanifolium Root is the dried root of Baliospermum solanifolium (Burm.) Suresh [B. axillare Blume, B. montanum (Willd.) Müll. Arg., Croton solanifolius Burm.] (Family Euphorbiaceae), Herbarium Specimen Number: DMSC 5310, Crude Drug Number: DMSc 1233.
Constituents Baliospermum Solanifolium Root contains phorbol esters (e.g., baliospermin, 12-deoxyphorbol-13-palmitate, and montanin) and propiophenones. It also contains triterpenoids, flavonoids, sterols, etc.
Description of the plant (Fig. 1) Monoecious (rarely dioecious) shrub 1 to 3 m tall; branched at base, young branch green, appressed pubescent, glabrous at maturity. Leaves simple, alternate, ovate to oblong, 8 to 21 cm long, 3 to 10 cm wide, apex obtuse or acute, base usually rounded, rarely cuneate, margin serrate or crenate, 2- to 5-lobed, chartaceous, glabrous or strigose on both surfaces, venation basally 3- to 5-nerved, nerves 6 to 8 pairs along midrib, basally with 2 glands; petiole 1 to 13 cm long. Inflorescence paniculate, axillary and terminal; male inflorescence 1 to 8(‒16) cm long; female inflorescence up to 1 cm long. Flower whitish green to yellowish; sepals 5 to 6, connate at base; petal absent. Male flowers many, 2 to 3 mm in diameter; pedicel 0.2 to 1.2 cm long; sepal orbicular to ovate, about 1.5 mm long, about 1 mm wide; disc annular, cup-shaped, 1 to 1.5 mm in diameter; stamens 10 to 12, filament 0.5 to 1 mm long, anther oblong. Female flowers 1 to 3, axillary or inserted at the base of male inflorescence, 2 to 4 mm in diameter; pedicel 0.2 to 1.1 cm long; sepal ovate or triangular, 1 to 2 mm long, 0.8 to 1 mm wide, externally pubescent; ovary superior, subglobose, 1 to 3 mm in diameter, pubescent, style stout, stigmas 3, widening into shortly split wings. Fruit a 3-lobed capsule, septicidally and loculicidally dehiscent, pendulous, subglobose, 0.8 to 1.3 cm in diameter, pubescent; calyx persistent, accrescent up to (3‒)5 mm long, up to 2(‒3) mm wide. Seed ovoid, 3 to 6 mm long and about 3 mm wide, marbled, carunculate.
Description Odour, mild; taste bland.
Macroscopical (Fig. 1) Pieces of cylindrical roots, some with lateral roots. Externally dark brown to greyish brown, longitudinally wrinkled or furrowed. Texture hard and tough; bark brown to dark brown or, wood pale yellow.
Microscopical (Figs. 2a, 2b) Transverse section of the root shows periderm and vascular tissue. Periderm: several layers of thin-walled rectangular cork cells. Vascular tissue: phloem tissue, polygonal parenchyma cells containing rosette aggregate crystals or starch grains, phloem ray, and laticifers; vascular cambium, several layers of thin-walled rectangular cells; xylem tissue, vessels, xylem rays, mostly uniseriate, occasionally 2- to 3-seriate, fibres, and parenchyma containing starch grains or rosette aggregate crystals.


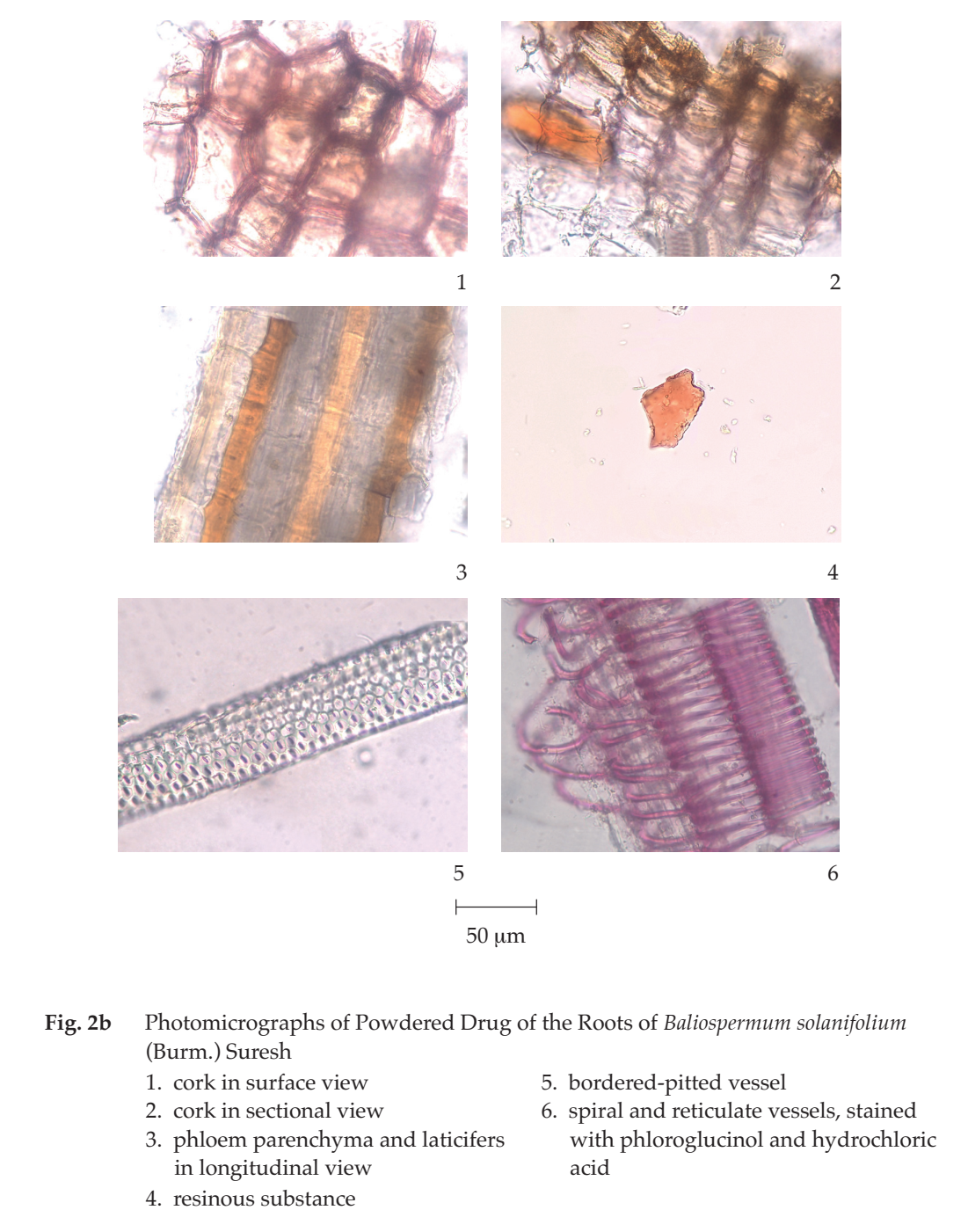
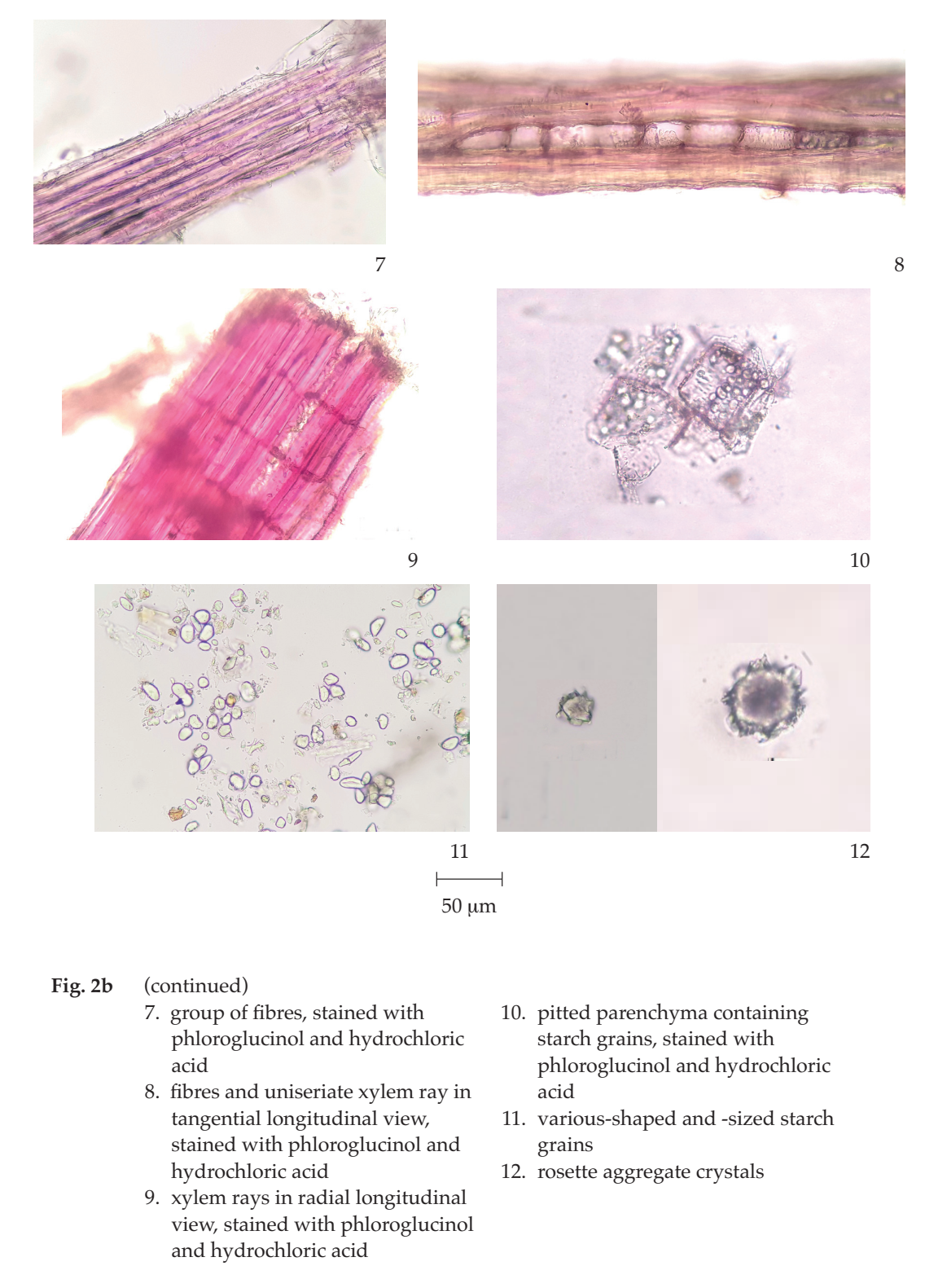
Baliospermum Solanifolium Root in powder possesses the diagnostic microscopical characters of the unground drug. Various-shaped and -sized starch grains, various-sized rosette aggregate crystals, pitted parenchyma containing starch grains, laticifers, and uniseriate xylem ray are characteristic.
Packaging and storage Baliospermum Solanifolium Root shall be kept in well-closed containers, protected from light, and stored in a dry place.
Identification
A. To 2 g of the sample, in fine powder, add 20 mL of ethanol, shake for 5 minutes and filter (solution 1). To 10 mL of solution 1, add 4 or 5 pieces of magnesium ribbon, shake well, and mix with a few drops of hydrochloric acid: a pale pink colour develops.
B. To 1 mL of solution 1, add a few drops of a 2.5 per cent w/v solution of iron(III) chloride and shake well: a black-blue colour develops.
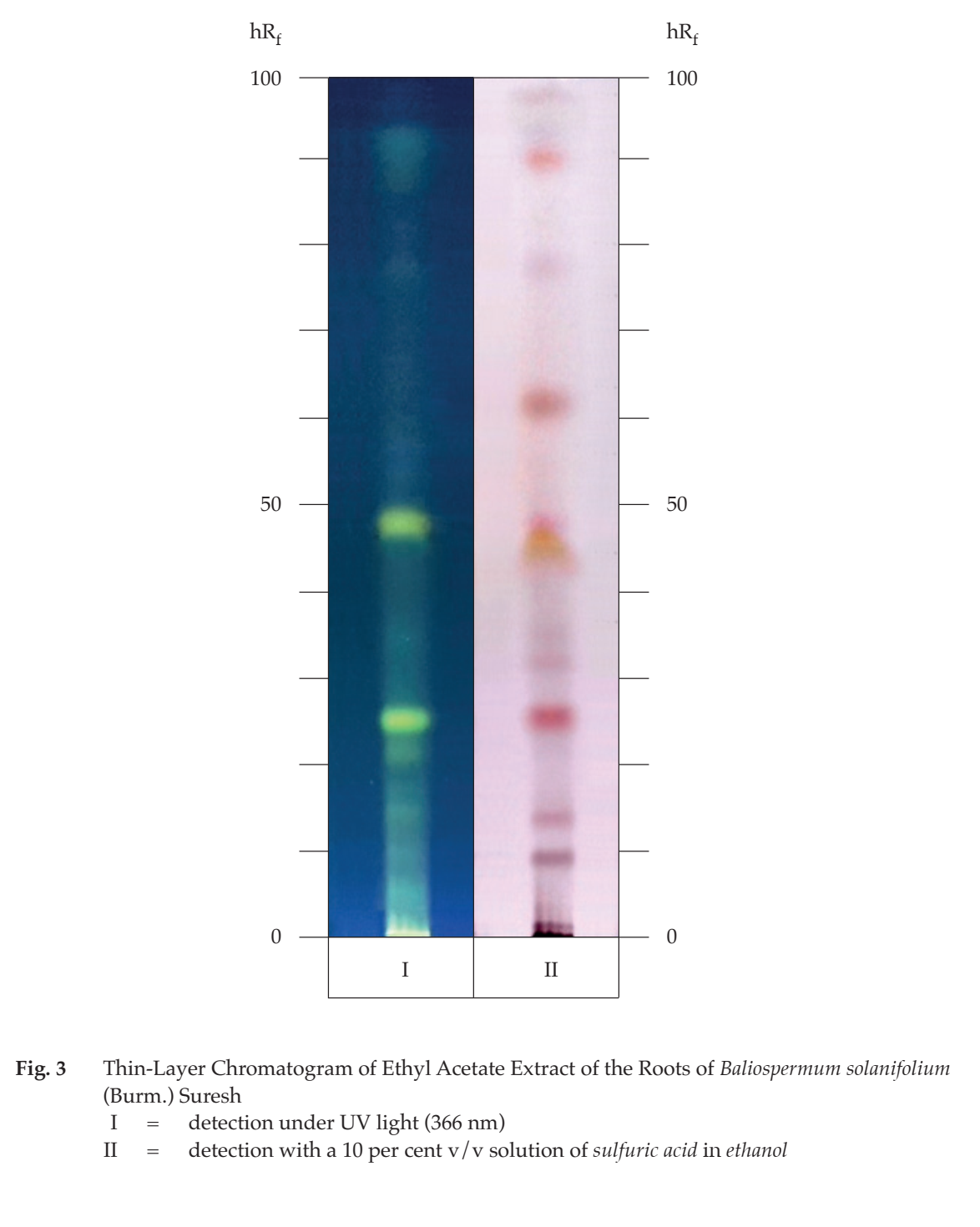
C. Carry out the test as described in the “Thin-Layer Chromatography” (Appendix 3.1), using silica gel 60 F254 as the coating substance and a mixture of 98 volumes of dichloromethane and 2 volumes of methanol as the mobile phase and allowing the solvent front to ascend 10 cm above the line of application. Apply to the plate as a band of 8 mm, 10 µL of the test solution prepared by refluxing 10 g of the sample, in fine powder, with 100 mL of ethyl acetate for 1 hour, allowing to cool for 30 minutes and filtering. Evaporate 5 mL of the filtrate to dryness and dissolve the residue in 1 mL of dichloromethane. After removal of the plate, allow it to dry in air and examine the plate under ultraviolet light (366 nm); three yellow and three blue fluorescent bands are observed. Then spray the plate with a 10 per cent v/v solution of sulfuric acid in ethanol and heat at 110° for 5 minutes; one brown and eight purple bands are observed (Fig. 3).
Loss on drying Not more than 8.0 per cent w/w after drying at 105° to constant weight (Appendix 4.15).
Foreign matter Not more than 2.0 per cent w/w (Appendix 7.2).
Acid-insoluble ash Not more than 3.0 per cent w/w (Appendix 7.6).
Total ash Not more than 8.0 per cent w/w (Appendix 7.7).
Ethanol-soluble extractive Not less than 1.0 per cent w/w (Appendix 7.12).
Water-soluble extractive Not less than 6.0 per cent w/w (Appendix 7.12).