ตำรามาตรฐานยาสมุนไพรไทย
Thai Herbal Pharmacopoeia
สำนักยาและวัตถุเสพติด กรมวิทยาศาสตร์การแพทย์ กระทรวงสาธารณสุข
Bureau of Drug and Narcotic, Department of Medical Sciences, Ministry of Public Health(Tinospora crispa (L.) Hook.f. & Thomson)
(Nelumbo nucifera Gaertn.)
(Centella asiatica (L.) Urb.)
(Centella Dry Extract)
(Centella Cream)
(Mesua ferrea L.)
(Piper sarmentosum Roxb.)
(Piper sarmentosum Roxb.)
(Pterocarpus santalinus L. f.)
(Santalum album L.)
(Senna tora (L.) Roxb.)
(Senna alata (L.) Roxb.)
(Senna Alata Tea)
(Piper retrofractum Vahl)
(Myristica fragrans Houtt)
(Andrographis paniculata (Burm. f.) Nees)
(Andrographis Capsules)
(Allium ascalonicum L.)
(Ocimum tenuiflorum L.)
(Curcuma longa L.)
(Turmeric Capsules)
(Turmeric Dry Extract)
(Turmeric Dry Extract Capsules)
(Arcangelisia flava (L.) Merr.)
(Curcuma sp.)
Harrisonia perforata (Blanco) Merr.
(Aristolochia pierrei Lecomte)
(Zingiber officinale Roscoe)
(Ginger Capsules)
(Ginger Tea)
(Cassia fistula L.)
(Nardostachys jatamansi (D. Don) DC.)
(Angelica sinensis (Oliv.) Diels)
Artemisia annua L.
(Ligusticum sinense Oliv. cv. Chuanxiong)
(Neopicrorhiza scrophulariiflora Pennell)
(Atractylodes lancea (Thunb.) DC.)
(Aucklandia lappa Decne)
(Terminalia chebula Retz.)
(Angelica dahurica (Hoffm.) Benth. & Hook. f. ex Franch. & Sav. var. dahurica)
(Kaempferia parviflora Wall. ex Baker)
(Hibiscus sabdariffa L.)
(Roselle Tea)
(Allium sativum L.)
(Zingiber zerumbet (L.) Sm.)
(Wurfbainia testacea (Ridl.) Škorničk.& A. D. Poulsen)
(Cannabis sativa L.)
(Myristica fragrans Houtt)
(Dracaena cochinchinensis (Lour.) S. C. Chen)
(Ficus racemosa L.)
(Hyptis suaveolens (L.) Poit.)
Clerodendrum indicum (L.) Kuntze
(Phyllanthus emblica L.)
(Citrus hystrix DC.)
(Citrus hystrix DC.)
(Areca catechu L.)
(Momordica charantia L.)
Moringa oleifera Lam.
(Aegle marmelos (L.) Corrêa)
(Solanum trilobatum L.)
(Morus alba L.)
Gynostemma pentaphyllum(Thunb.)
Makino
(Clinacanthus nutans (Burm. f.) Lindau)
(Cissus quadrangularis L.)
(Mimusops elengi L.)
(Zingiber montanum (J. König) Link. ex A. Dietr.)
(Piper betle L.)
(Capsicum annuum L.)
(Capsicum Oleoresin)
(Capsicum Gel)
(Piper nigrum L.)
(Piper nigrum L.)
(Eurycoma longifolia Jack)
(Thunbergia laurifolia Lindl.)
(Piper wallichii (Miq.) Hand.-Mazz.)
Senna garrettiana (Craib) H. S. Irwin & Barneby
(Terminalia bellirica (Gaertn.) Roxb.)
(Terminalia chebula Retz.)
(Caesalpinia bonduc (L.) H. Roxb.)
(Tarlmounia elliptica (DC.) H. Rob., S. C. Keeley, Skvaria & R. Chan)
(Hog Creeper Vine Dry Extract Capsiles)
(Hog Creeper Vine Dry Extract)
(Brachypterum scandens (Roxb.) Miq.)
(Lepidium sativum L.)
(Nigella sativa L.)
(Cuminum cyminum L.)
(Foeniculum vulgare Mill.)
(Plantago ovata Forssk.)
(Pimpinella anisum L.)
(Carum carvi L.)
(Anethum graveolens L.)
(Trachyspermum ammi (L.) Sprague)
Albizia procera (Roxb.) Benth.
(Acorus calamus L.)
(Tiliacora triandra (Colebr.) Diels)
Cyanthillium cinereum (L.) H. Rob.
(Orthosiphon aristatus (Blume) Miq.)
Murdannia loriformis (Hassk.) R. S. Rao & Kammathy
(Capparis micracantha DC.)
(Chrysopogon zizanioides (L.) Roberty)
(Cyperus rotundus L.)
(Cannabis sativa L.)
(Syzygium aromaticum (L.) Merr. & L. M. Perry)
(Boesenbergia rotunda (L.) Mansf.)
(Acanthus ebracteatus Vahl)
(Acanthus ilicifolius L.)
(Kaempferia galanga L.)
(Curcuma comosa Roxb.)
Betula alnoides Buch.-Ham. ex D. Don
Cannabis sativa L.
Carthamus tinctorius L
Mitragyna speciosa (Korth.) Havil
Mallotus repandus (Rottler) Müll. Arg
Azadirachta indica A. Juss. var. siamensis Valeton
Azadirachta indica A. Juss. var. siamensis Valeton
Punica granatum L.
Rhinacanthus nasutus (L.) Kurz
Baliospermum solanifolium (Burm.) Suresh
Curcuma aeruginosa Roxb
Boesenbergia kingii Mood & L. M. Prince
Senegalia rugata (Lam.) Britton & Rose
Acacia concinna (Willd.) DC.
Senegalia rugata (Lam.) Britton & Rose
Acacia concinna (Willd.) DC.
Senna alexandriana Mill. var. alexandriana
Cassia acutifolia Delile, Cassia angustifolia Vahl
Butea superba Roxb. ex Willd.
[Plaso superba (Roxb. ex Willd.) Kuntze, Rudolphia superba (Roxb. ex Willd.) Poir.
Pueraria candollei Graham
ex Benth. var. mirifica (Airy Shaw & Suvat.) Niyomdham
Streblus asper Lour.
Suregada multiflora (A. Juss.) Baill. (Gelonium
multiflorum A. Juss.
Cannabis is the dried female flowering top of Cannabis sativa L. [C. indica Lam., C. ruderalis Janisch., C. ruderalis (Janisch.) S. Z. Liou, C. sativa subsp. indica (Lam.) E. Small & Cronquist, C. sativa var. indica (Lam.) Wehmer] (Family Cannabaceae), Herbarium Specimen Number: DMSC 5268, Crude Drug Number DMSc 1188.
Constituents Cannabis contains cannabinoids (e.g., cannabidiol or CBD, Δ9-tetrahydrocannabinol or Δ9-THC or THC). It also contains monoterpenes (e.g.,
d-limonene, β-myrcene, α-pinene, γ-terpinolene), sesquiterpenes (e.g., β-caryophyllene, α-humulene), etc.
Description of the plant (Fig. 1) Herbaceous plant, dioecious, occasionally monoecious, 0.2 to 6 m tall; stem and branches angular, erect, covered with rather short stiff hairs. Leaves palmately compound, opposite near base, spirally arranged upwards; petiole up to 10 cm long, pubescent; stipule erect, linear or narrowly subulate, 4 to 6 mm long, persistent; leaflets (3‒) 5 to 11, narrowly lanceolate, linear to linear-lanceolate, 2 to 15 cm long, 0.2 to 2 cm wide, apex acuminate-caudate, base narrowly cuneate, margin coarsely serrate, upper surface dark green, scabrous, lower surface whitish green with scattered brownish resinous dots, densely pubescent with appressed hairs, nerves 8 to 20 pairs; sessile. Flowering top numerous inflorescences, terminal or axillary; male inflorescence diffused and paniculate, bracts small; female inflorescence, crowded among foliaceous bracts, 1 to 5 cm long. Flower small, unisexual; male flower: yellowish green, tepals 5, free, elliptic or oblong, 3 to 5 mm long, 1.5 to 2 mm wide, apex acute, margin entire, finely pubescent; stamens 5, opposite tepals, filament slender, 0.5 to 1 mm long, anther basifixed, oblong, 2-celled, dehiscing by apical pore; female flower: greenish, subsessile, enveloped by perigonal bract, membranous spathe-like, dark green, with glandular hairs; tepals fused into thin-textured tube adnate to ovary, in some cultivars, merely a ring at base of ovary; ovary superior, subglobose, 1 to 2 mm in diameter, 1-loculed, style short divided into 2 stigmatic slender arms, brown, pubescent, caducous. Fruit an achene, ovoid or ellipsoid, 2 to 5 mm long, 3 to 4 mm wide, somewhat compressed, minutely pilose to glabrous, shiny, yellowish brown to white or greenish, mottled with purple pericarp crustaceous, finely reticulate. Seed 1.
Description Odour, sweet, ranging from intense smell of ripe mango, orange peel, to minty in fresher specimens, to odourless in older samples; taste, bland, with slightly bitter aftertaste.
Macroscopical (Fig. 1) Cannabis occurs as stem, leaves, and female inflorescences, light green to brownish. Stem, angular, covered with rather short, stiff hairs. Leaves, palmately compound or reduced to simple. Female inflorescence, crowded among foliaceous bracts, 1 to 5 cm long; female flower, enveloped by perigonal bract, membranous spathe-like, dark green, with glandular hairs; tepals fused into thin-textured tube adnate to ovary, in some cultivars; ovary, subglobose, minute.
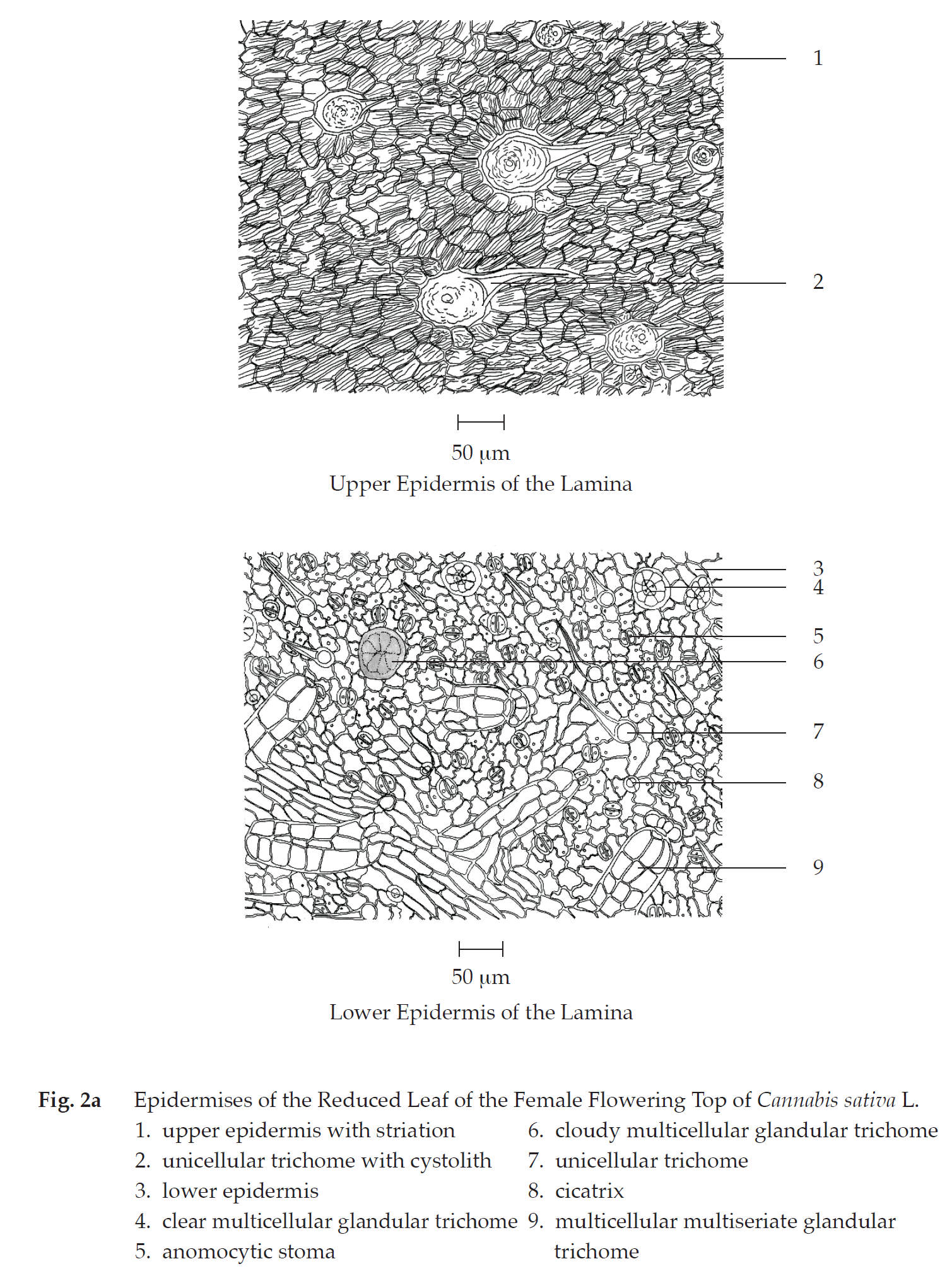
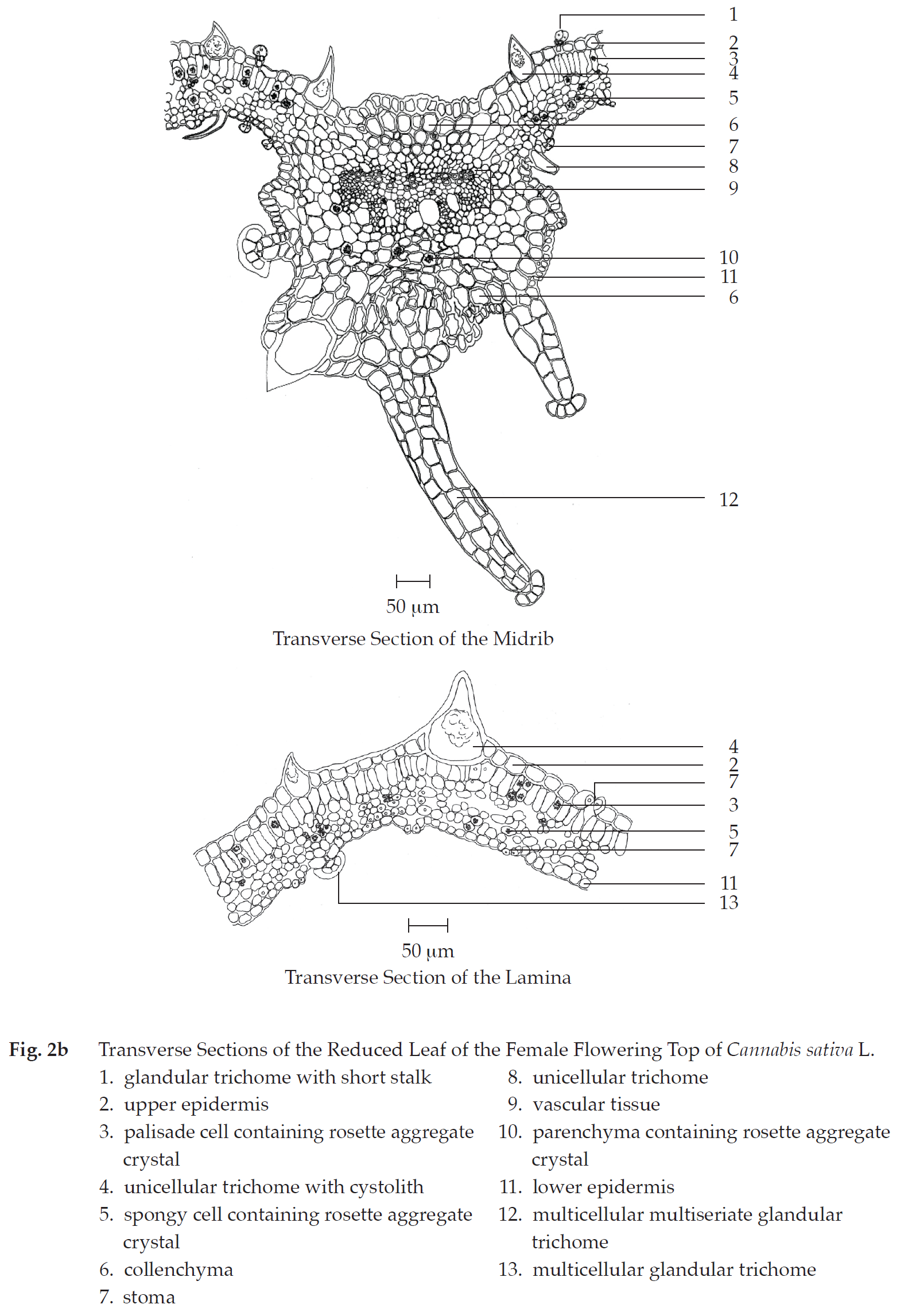
Microscopical (Figs. 2a, 2b, 2c, 2d, 2e, 2f, 2g, 2h) Transverse section of the reduced leaf of the female flowering top through the midrib and the lamina shows upper epidermis, mesophyll, vascular tissue, and lower epidermis. Upper epidermis: a layer of rectangular cells, covered with cuticle layer; stomata; unicellular trichomes with cystolith; glandular trichomes with short stalk. Mesophyll: a layer of cylindrical palisade cells, some containing rosette aggregate crystals; spongy cells, irregularly shaped, loosely arranged, some containing rosette aggregate crystals; collenchyma, angular and lacunar; and parenchyma, some containing rosette aggregate crystals, occurring in the upper and lower parts of the midrib. Vascular tissue: phloem and xylem, scattering in the mesophyll. Lower epidermis:
a layer of small rectangular cells, covered with cuticle layer; stomata; unicellular trichomes; glandular trichomes with short stalk; multicellular multiseriate glandular trichomes.
In surface view, the lamina shows upper epidermis, slightly rectangular cells withstriation, unicellular trichomes with cystolith and cicatrix; lower epidermis, slightly wavy rectangular cells, anomocytic stomata, cicatrices, unicellular trichomes, clear or cloudy multicellular glandular trichomes, and multicellular multiseriate glandular trichomes.
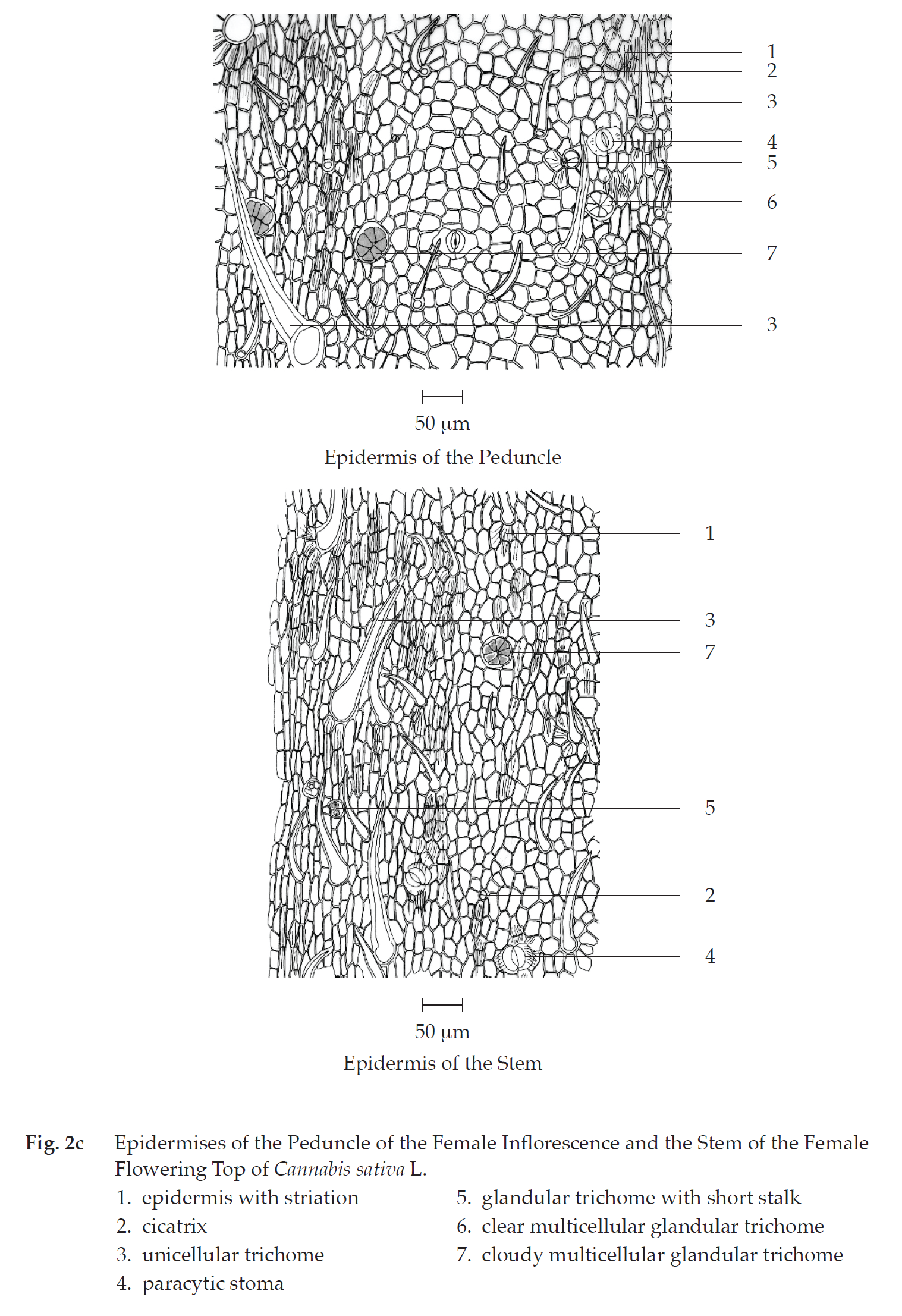
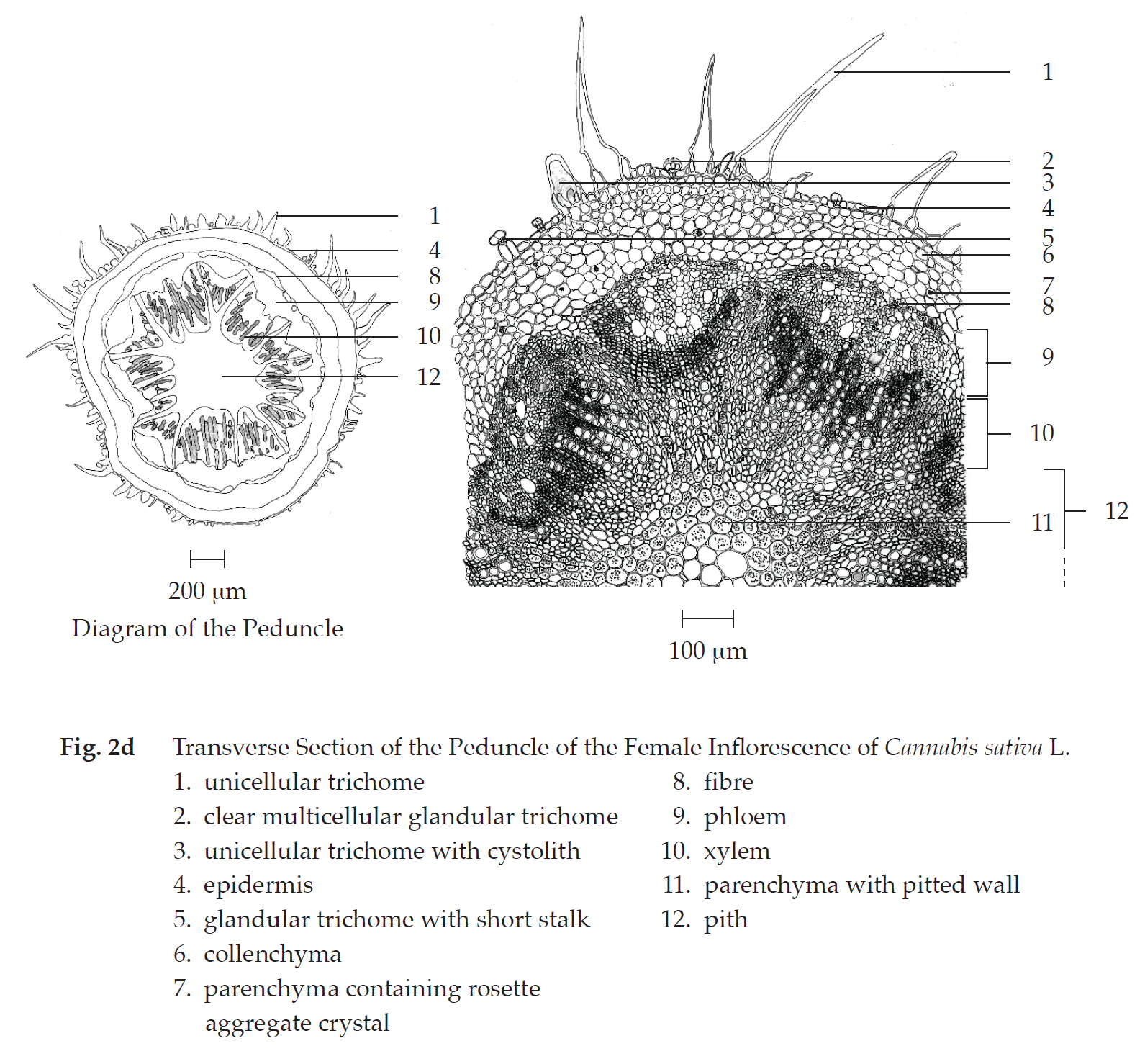
Transverse section of the peduncle of the female inflorescence shows epidermis, cortex, vascular tissue, and pith. Epidermis: a layer of rectangular cells, covered with cuticle layer; stomata; unicellular trichomes and unicellular trichomes with cystolith; clear or cloudy glandular trichomes with or without short stalk. Cortex: collenchyma, lacunar; parenchyma, some containing rosette aggregate crystals. Vascular tissue: collateral vascular bundles, phloem and xylem; groups of fibres. Pith: parenchyma with reticulate or pitted wall and thin-walled parenchyma, some containing rosette aggregate crystals.
In surface view, the peduncle shows polygonal epidermal cells, some with striation, cicatrices, paracytic stomata, unicellular trichomes, glandular trichomes with short stalk, and clear or cloudy multicellular glandular trichomes.
Transverse section of the stem of the female flowering top shows epidermis, cortex, vascular tissue, and pith. Epidermis: a layer of small rectangular cells, covered with cuticle layer; stomata; unicellular trichomes; clear or cloudy glandular trichomes with or without short stalk; multicellular trichomes. Cortex: collenchyma, lacunar; parenchyma, some containing rosette aggregate crystals and brown substance; groups of fibres. Vascular tissue: collateral vascular bundles, phloem and xylem. Pith: parenchyma with reticulate or pittedwalled parenchyma, some containing rosette aggregate crystals.
In surface view, the stem shows polygonal epidermal cells, some with striation, unicellular trichomes, glandular trichomes with short stalk, cloudy multicellular glandular trichomes and cicatrices.
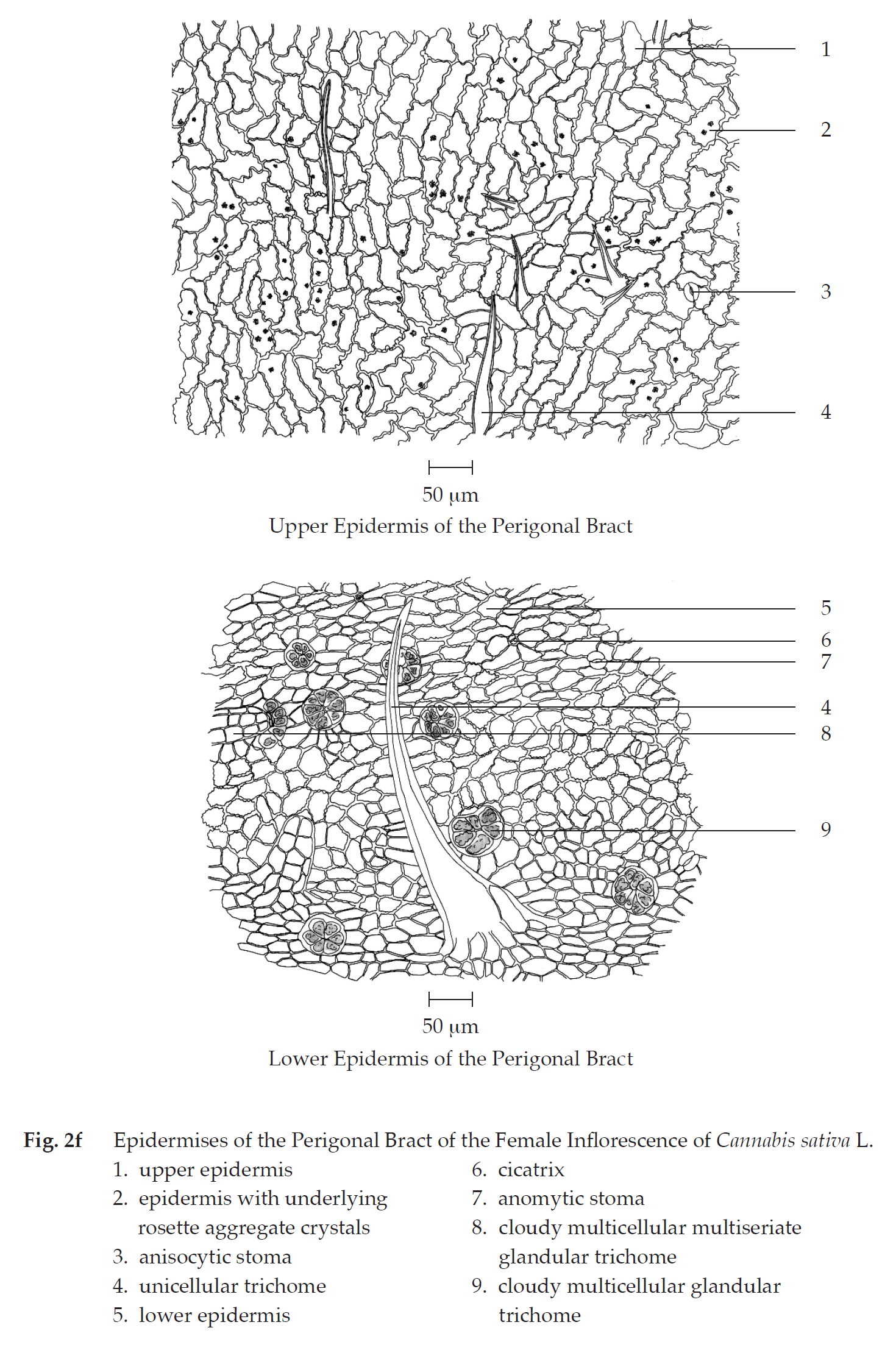
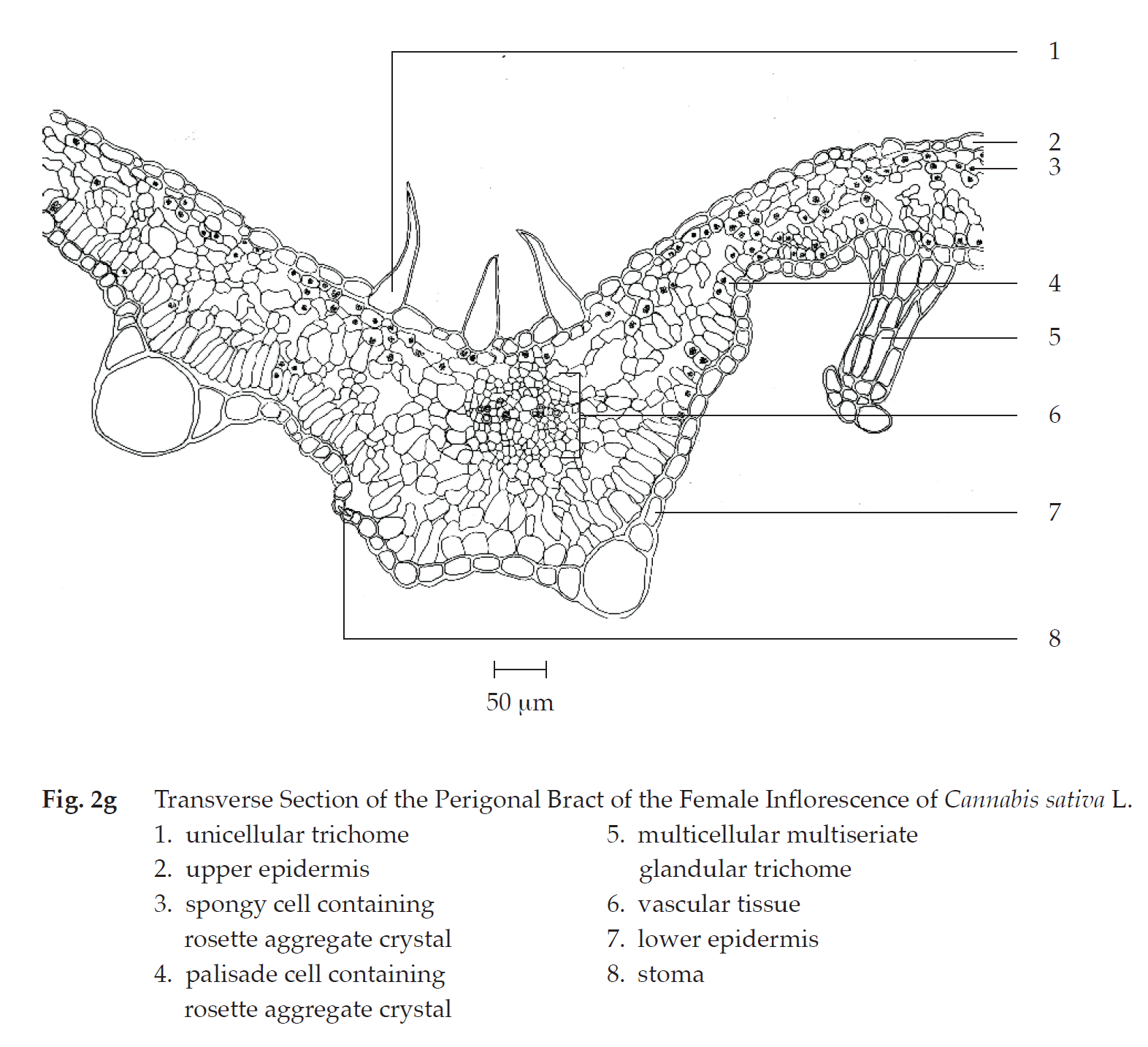
Transverse section of the perigonal bract of the female inflorescence shows upper epidermis, mesophyll, vascular tissue, and lower epidermis. Upper epidermis: a layer of rectangular cells; unicellular trichomes and unicellular trichomes with cystolith; glandular trichomes with short stalk. Mesophyll: a layer of cylindrical palisade cells, some containing rosette aggregate crystals, adjacent to lower epidermis; spongy cells, loosely arranged, some containing rosette aggregate crystals, adjacent to upper epidermis. Vascular tissue: phloem and xylem, located in the mesophyll. Lower epidermis: a layer of rectangular cells; stomata; unicellular trichomes and unicellular trichomes with cystolith; clear or cloudy multicellular multiseriate glandular trichomes.
In surface view, the perigonal bract shows slightly wavy upper epidermal cells, varying in size and shape, some with underlying rosette aggregate crystals, anisocytic stomata, and unicellular trichomes; slightly wavy lower epidermis, varying in size and shape, anomocytic stomata, cicatrices, cloudy multicellular glandular trichomes, and cloudy multicellular multiseriate glandular trichomes.


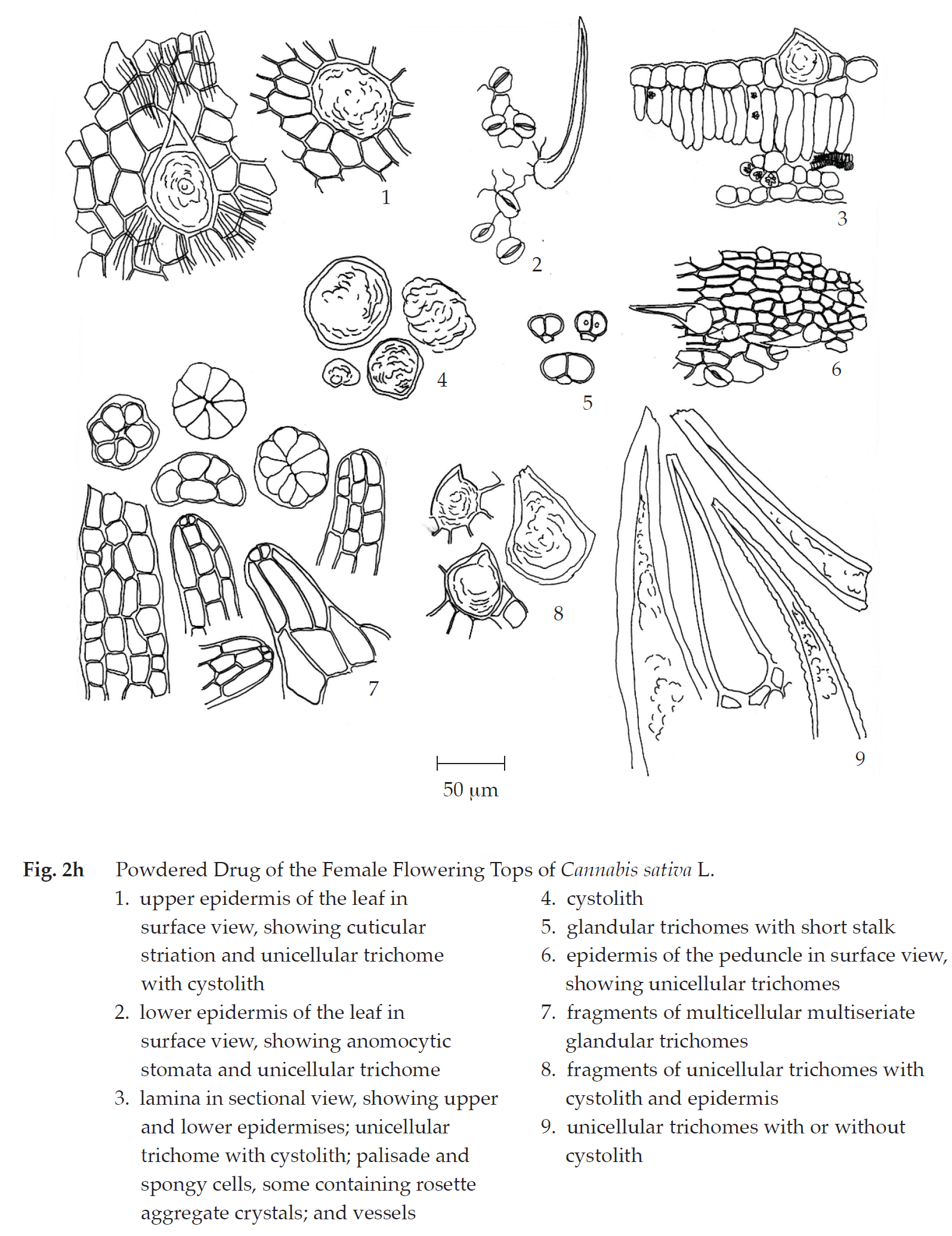
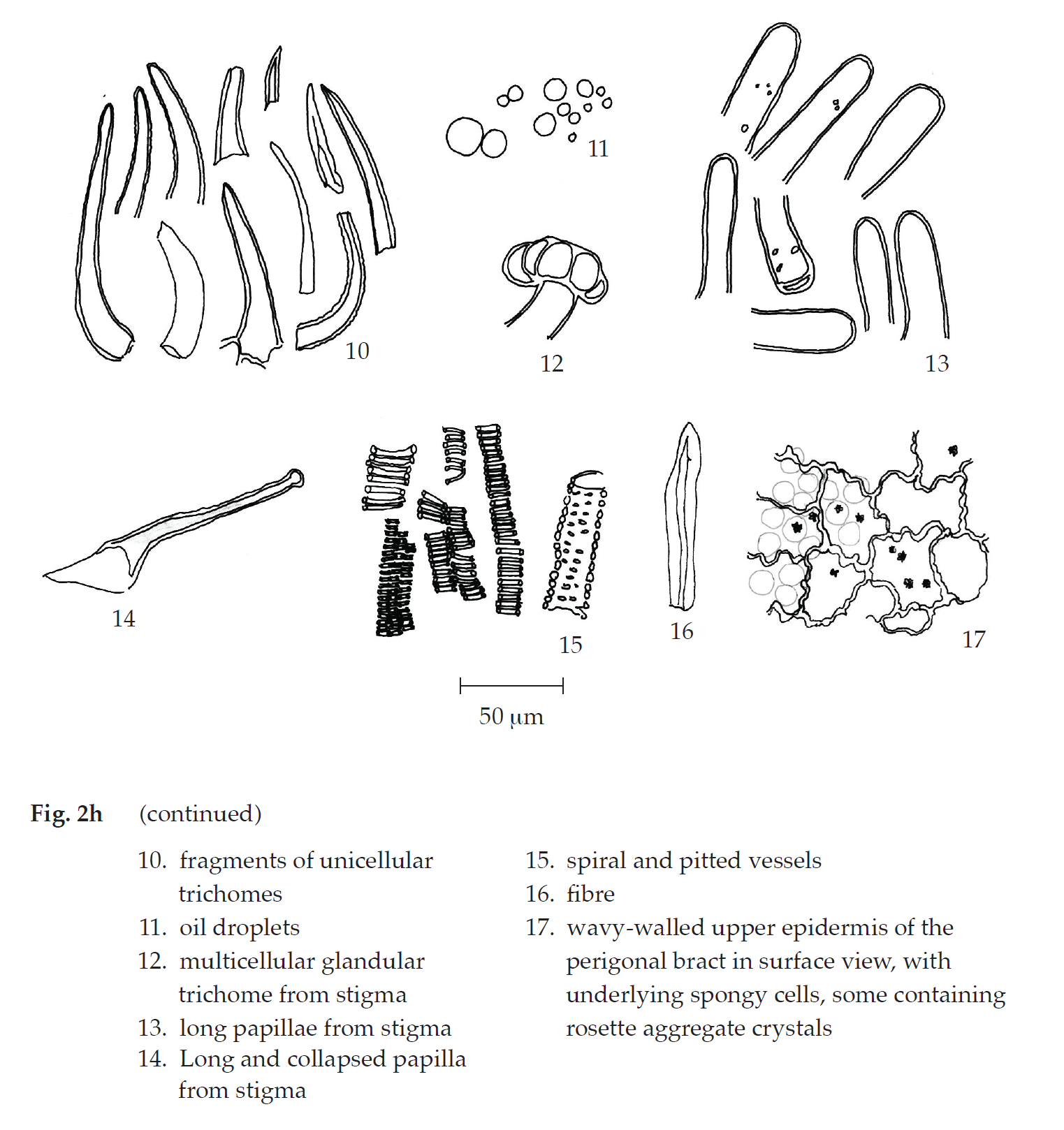
Cannabis in powder possesses the diagnostic microscopical characters of the unground drug. Unicellular trichomes and multicellular multiseriate glandular trichomes can be seen in abundance. However, unicellular trichomes with cystolith, together with glandular trichomes with short stalk, are unique. Long papillae, long and collapsed papillae, and multicellular glandular trichomes, all of which from stigmas, together with wavy-walled upper epidermis of perigonal bracts are found.
Additional information
1. It is commonly used with other herbal drugs in Thai traditional herbal preparations.
2. It is considered a narcotic under Category 5 of the Narcotics Act B.E. 2522 (1979).
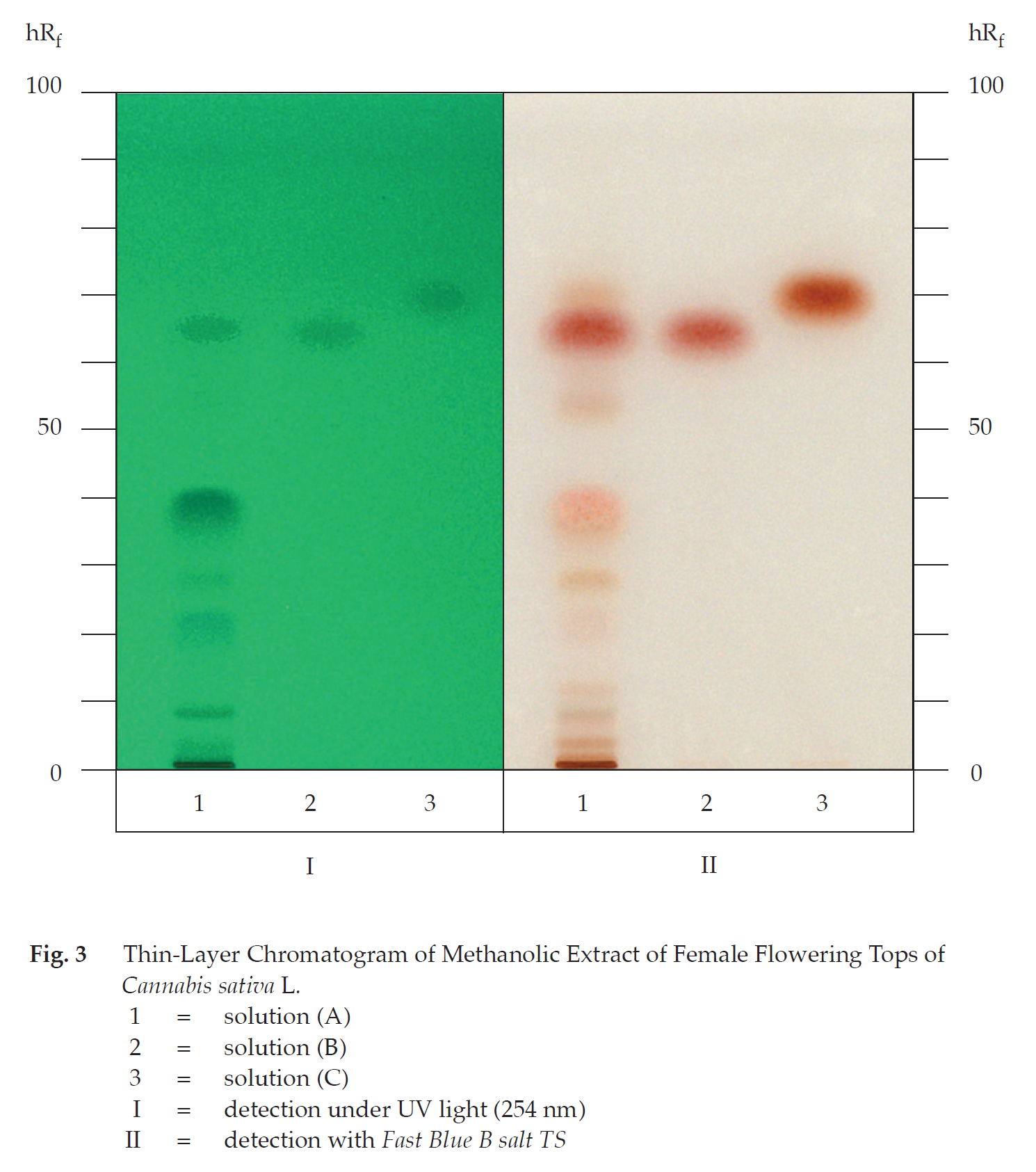
3. It is to be noted that the TLC patterns may vary due to variation in the CBD and THC contents in different cultivars of the cannabis samples.
4. Cannabis plants are ready to harvest when about 75 per cent of the stigmas turn brown.
5. The two major cannabinoids found in cannabis, Δ9-tetrahydrocannabinol (Δ9-THC or THC) and cannabidiol (CBD), are initially produced by cannabis in their carboxylic acid forms which are Δ9-tetrahydrocannabinolic acid (THCA) and cannabidiolic acid (CBDA), respectively, before converted into Δ9-THC and CBD by heating (e.g., by smoking or baking), light or natural degradation. The conversion process is known as decarboxylation. Δ9-THC is the cannabinoid predominantly responsible for the psychoactive properties of cannabis.
6. Some preparations from a total of 16 cannabis-containing preparations stated under the Notification of the Ministry of Public Health, Re: Prescription of Narcotic Drug Preparations of Category 5 Containing Cannabis for Medical Uses or for Research Purpose B.E. 2562 (2019) may potentially have analgesic effect.
Packaging and storage Cannabis shall be kept in well-closed containers, protected from light, and stored in a cool and dry place.
Identification
A. Macerate 100 mg of the sample, in powder, with 10 mL of petroleum ether (boiling range, 60° to 80°) for 10 minutes and filter. Evaporate 1 mL of the filtrate to dryness. Dissolve the residue in 1 mL of methanol. Add 1 drop of Fast Blue B salt TS and 1 drop of a 10 per cent w/v solution of sodium hydrogen carbonate: an orange-red colour is produced.
B. The chromatogram of the Sample preparation shows several peaks, one of which corresponds to the tetrahydrocannabinol peak of the Standard preparations, as obtained in the Tetrahydrocannabinol content.
C. Carry out the test as described in the “Thin-layer Chromatography” (Appendix 3.1), using a high-performance plate with silica gel 60 F254 as the coating substance and a mixture of 80 volumes of petroleum ether (boiling range, 60° to 80°) and 20 volumes of ether as the mobile phase and allowing the solvent front to ascend 8 cm above the line of application. Apply separately to the plate, as bands of 7 mm, 10 μL of solution (A) and 5 μL each of solutions (B) and (C). For solution (A), macerate 100 mg of the sample, in powder, with 5 mL of methanol, shake for 10 minutes and filter. Solution (B) contains 1 mg per mL of Tetrahydrocannabinol RS in ethanol (Lipomed® or equivalent is suitable.). For solution (C), dissolve 1 mg of Cannabidiol RS in 1 mL of methanol. After removal of the plate, allow it to dry in air and examine under ultraviolet light (254 nm), marking the quenching bands. The chromatogram obtained from solution (A) shows two quenching bands (hRf values 62 to 68 and 69 to 73), corresponding to the tetrahydrocannabinol and the cannabidiol bands from solutions (B) and (C), respectively. Subsequently, spray the plate with Fast Blue B salt TS and examine under visible light. The chromatogram obtained from solution (A) shows a red band due to tetrahydrocannabinol and an orange band due to cannabidiol. Several other bands of red colour are also observed (Table 1); see also Fig. 3.
Table 1 hRf Values of Components in Methanolic Extract of the Female Flowering Tops of Cannabis sativa L.
| Band | hRf Value | Detection | |
| UV 254 | Fast Blue B salt TS | ||
| 1 | 3-5 | weak quenching | pale red |
| 2 | 7-9 | quenching | pale red |
| 3 | 11-13 | - | pale red |
| 4 | 19-24 | quenching | pale red |
| 5 | 27-30 | weak quenching | pale red |
| 6 | 35-43 | quenching | pale red |
| 7 | 52-56 | - | pale red |
| 8* | 62-68 | quenching | red |
| 9** | 69-73 | weak quenching | pale orange |
*tetrahydrocannabinol
**cannabidiol
Loss on drying Not more than 9.0 per cent w/w after drying at 105° to constant weight (Appendix 4.15).
Foreign matter Not more than 5.0 per cent w/w of stems 3 mm or more in diameter and not more than 2.0 per cent w/w of other foreign matter (e.g., fruits) (Appendix 7.2).
Acid-insoluble ash Not more than 3.0 per cent w/w (Appendix 7.6).
Total ash Not more than 15.0 per cent w/w (Appendix 7.7).
Ethanol-soluble extractive Not less than 14.0 per cent w/w (Appendix 7.12).
Tetrahydrocannabinol content Not less than 1.0 per cent w/w of tetrahydrocannabinol (C21H30O2). Carry out the determination as described in the “Liquid Chromatography” (Appendix 3.5).
Mobile phase A Use acetonitrile.
Mobile phase B Prepare a 0.1 per cent v/v solution of trifluoroacetic acid.
Standard stock preparation Use a solution containing 1 mg per mL of Tetrahydrocannabinol RS in ethanol (Lipomed® or equivalent is suitable.).
Standard preparations Transfer 1.0 mL of Standard stock preparation to a 50-mL volumetric flask, dilute with acetonitrile to volume and mix. Dilute the solution quantitatively and stepwise with the same solvent to obtain five solutions having known concentrations of 2, 4, 6, 8, and 10 μg per mL.
Sample preparation Sonicate about 200 mg of Cannabis, in fine powder and accurately weighed, in 20.0 mL of acetonitrile for 20 minutes and filter. Transfer 1.0 mL of the filtrate to a 20-mL volumetric flask, dilute to volume with acetonitrile and mix. Filter through a nylon membrane having a 0.45-μm porosity.
The step gradient of mobile phases is as follows:
| Time (Minutes) |
Mobile Phase A (Per Cent V/V) |
Mobile Phase B (Per Cent V/V) |
| 0 | 55 | 45 |
| 1 | 55 | 45 |
| 9 | 100 | 0 |
| 11 | 100 | 0 |
| 13 | 55 | 45 |
Chromatographic system The chromatographic procedure may be carried out using (a) a stainless steel column (15 cm × 4.6 mm) packed with octadecylsilane chemically bonded to porous silica or ceramic microparticles (2.7 μm), equipped with a similarly packed guard column (5 mm × 3.9 mm) and maintained at a temperature of 35°±1°, (b) Mobile phase at a flow rate of about 1.8 mL per minute, and (c) an ultraviolet photometer set at 228 nm.
To determine the suitability of the chromatographic system, chromatograph Standard preparation having a known concentration of 6 μg per mL, and record the peak response as directed under Procedure and Calculation: the relative standard deviation for replicate injections is not more than 2.0 per cent.
Procedure and Calculation Separately inject about 25 μL each of Standard preparations into the chromatograph, record the chromatograms and measure the responses for tetrahydrocannabinol peaks. Plot the readings and draw the standard curve of best fit: the curve shows the correlation coefficient of not less than 0.999. Inject about 25 μL of Sample preparation into the chromatograph, record the chromatogram and measure the response for tetrahydrocannabinol peak. By reference to the standard curve, calculate the content of tetrahydrocannabinol (C21H30O2) in the portion of the Cannabis taken.