ตำรามาตรฐานยาสมุนไพรไทย
Thai Herbal Pharmacopoeia
สำนักยาและวัตถุเสพติด กรมวิทยาศาสตร์การแพทย์ กระทรวงสาธารณสุข
Bureau of Drug and Narcotic, Department of Medical Sciences, Ministry of Public Health(Tinospora crispa (L.) Hook.f. & Thomson)
(Nelumbo nucifera Gaertn.)
(Centella asiatica (L.) Urb.)
(Centella Dry Extract)
(Centella Cream)
(Mesua ferrea L.)
(Piper sarmentosum Roxb.)
(Piper sarmentosum Roxb.)
(Pterocarpus santalinus L. f.)
(Santalum album L.)
(Senna tora (L.) Roxb.)
(Senna alata (L.) Roxb.)
(Senna Alata Tea)
(Piper retrofractum Vahl)
(Myristica fragrans Houtt)
(Andrographis paniculata (Burm. f.) Nees)
(Andrographis Capsules)
(Allium ascalonicum L.)
(Ocimum tenuiflorum L.)
(Curcuma longa L.)
(Turmeric Capsules)
(Turmeric Dry Extract)
(Turmeric Dry Extract Capsules)
(Arcangelisia flava (L.) Merr.)
(Curcuma sp.)
Harrisonia perforata (Blanco) Merr.
(Aristolochia pierrei Lecomte)
(Zingiber officinale Roscoe)
(Ginger Capsules)
(Ginger Tea)
(Cassia fistula L.)
(Nardostachys jatamansi (D. Don) DC.)
(Angelica sinensis (Oliv.) Diels)
Artemisia annua L.
(Ligusticum sinense Oliv. cv. Chuanxiong)
(Neopicrorhiza scrophulariiflora Pennell)
(Atractylodes lancea (Thunb.) DC.)
(Aucklandia lappa Decne)
(Terminalia chebula Retz.)
(Angelica dahurica (Hoffm.) Benth. & Hook. f. ex Franch. & Sav. var. dahurica)
(Kaempferia parviflora Wall. ex Baker)
(Hibiscus sabdariffa L.)
(Roselle Tea)
(Allium sativum L.)
(Zingiber zerumbet (L.) Sm.)
(Wurfbainia testacea (Ridl.) Škorničk.& A. D. Poulsen)
(Cannabis sativa L.)
(Myristica fragrans Houtt)
(Dracaena cochinchinensis (Lour.) S. C. Chen)
(Ficus racemosa L.)
(Hyptis suaveolens (L.) Poit.)
Clerodendrum indicum (L.) Kuntze
(Phyllanthus emblica L.)
(Citrus hystrix DC.)
(Citrus hystrix DC.)
(Areca catechu L.)
(Momordica charantia L.)
Moringa oleifera Lam.
(Aegle marmelos (L.) Corrêa)
(Solanum trilobatum L.)
(Morus alba L.)
Gynostemma pentaphyllum(Thunb.)
Makino
(Clinacanthus nutans (Burm. f.) Lindau)
(Cissus quadrangularis L.)
(Mimusops elengi L.)
(Zingiber montanum (J. König) Link. ex A. Dietr.)
(Piper betle L.)
(Capsicum annuum L.)
(Capsicum Oleoresin)
(Capsicum Gel)
(Piper nigrum L.)
(Piper nigrum L.)
(Eurycoma longifolia Jack)
(Thunbergia laurifolia Lindl.)
(Piper wallichii (Miq.) Hand.-Mazz.)
Senna garrettiana (Craib) H. S. Irwin & Barneby
(Terminalia bellirica (Gaertn.) Roxb.)
(Terminalia chebula Retz.)
(Caesalpinia bonduc (L.) H. Roxb.)
(Tarlmounia elliptica (DC.) H. Rob., S. C. Keeley, Skvaria & R. Chan)
(Hog Creeper Vine Dry Extract Capsiles)
(Hog Creeper Vine Dry Extract)
(Brachypterum scandens (Roxb.) Miq.)
(Lepidium sativum L.)
(Nigella sativa L.)
(Cuminum cyminum L.)
(Foeniculum vulgare Mill.)
(Plantago ovata Forssk.)
(Pimpinella anisum L.)
(Carum carvi L.)
(Anethum graveolens L.)
(Trachyspermum ammi (L.) Sprague)
Albizia procera (Roxb.) Benth.
(Acorus calamus L.)
(Tiliacora triandra (Colebr.) Diels)
Cyanthillium cinereum (L.) H. Rob.
(Orthosiphon aristatus (Blume) Miq.)
Murdannia loriformis (Hassk.) R. S. Rao & Kammathy
(Capparis micracantha DC.)
(Chrysopogon zizanioides (L.) Roberty)
(Cyperus rotundus L.)
(Cannabis sativa L.)
(Syzygium aromaticum (L.) Merr. & L. M. Perry)
(Boesenbergia rotunda (L.) Mansf.)
(Acanthus ebracteatus Vahl)
(Acanthus ilicifolius L.)
(Kaempferia galanga L.)
(Curcuma comosa Roxb.)
Betula alnoides Buch.-Ham. ex D. Don
Cannabis sativa L.
Carthamus tinctorius L
Mitragyna speciosa (Korth.) Havil
Mallotus repandus (Rottler) Müll. Arg
Azadirachta indica A. Juss. var. siamensis Valeton
Azadirachta indica A. Juss. var. siamensis Valeton
Punica granatum L.
Rhinacanthus nasutus (L.) Kurz
Baliospermum solanifolium (Burm.) Suresh
Curcuma aeruginosa Roxb
Boesenbergia kingii Mood & L. M. Prince
Senegalia rugata (Lam.) Britton & Rose
Acacia concinna (Willd.) DC.
Senegalia rugata (Lam.) Britton & Rose
Acacia concinna (Willd.) DC.
Senna alexandriana Mill. var. alexandriana
Cassia acutifolia Delile, Cassia angustifolia Vahl
Butea superba Roxb. ex Willd.
[Plaso superba (Roxb. ex Willd.) Kuntze, Rudolphia superba (Roxb. ex Willd.) Poir.
Pueraria candollei Graham
ex Benth. var. mirifica (Airy Shaw & Suvat.) Niyomdham
Streblus asper Lour.
Suregada multiflora (A. Juss.) Baill. (Gelonium
multiflorum A. Juss.
Plumbago zeylanica L.
Plumbago indica L.
Biancaea sappan (L.) Tod.
Ziziphus attopensis Pierre
Streblus asper Lour.
Justicia gendarussa Burm. f.
Enhalus acoroides (L. f.) Royle
Bridelia ovata Decne.
Tamarindus indica L.
Citrus × aurantiifolia (Christm.) Swingle
Garcinia mangostana L.
Blumea balsamifera (L.) DC
Persicaria odorata (Lour.) Soják
Zingiber montanum (J. König) Link ex A. Dietr.
Mammea siamensis (Miq.) T. Anderson
Citrus maxima (Burm.) Merr.
Citrus × aurantium L. ‘Som Sa’
Punica granatum L.
Rhinacanthus nasutus (L.) Kurz
Clinacanthus Nutans Leaf is the dried leaf of Clinacanthus nutans (Burm. f.) Lindau (Family Acanthaceae), Herbarium Specimen Number: DMSC 5137, BKF 161293, Crude Drug Number: DMSc 0727.
Constituents Clinacanthus Nutans Leaf contains glycolipids, phaeophytins, triterpenoids, sterols, C-glycoside flavones, cerebrosides, sulfur-containing glucosides, etc.
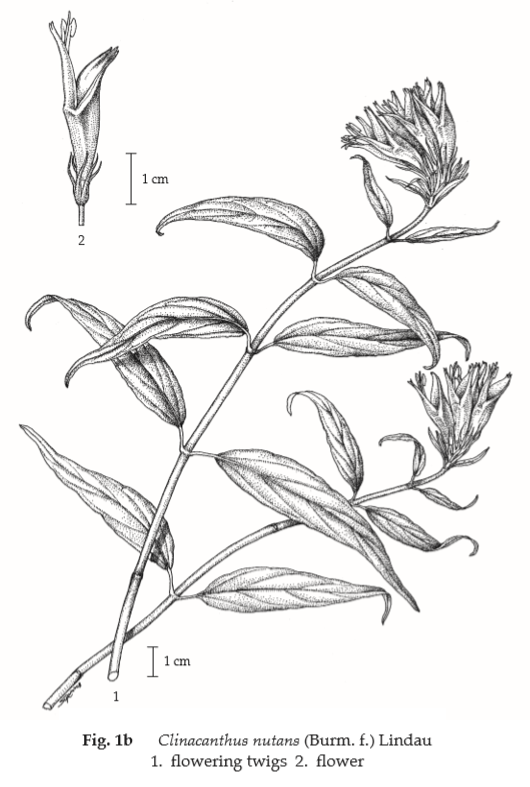
Description of the plant (Figs. 1a, 1b) Scandent shrub 1 to 3 m tall, erect-drooping or clambering; branchlets finely pubescent. Leaves simple, decussate, narrowly elliptic, oblong, ovate or lanceolate, 2.5 to 13 cm long, 0.5 to 3.5 cm wide, apex long-acuminate, base obtuse, rounded, or truncate, often oblique, margin exsculptate-dentate or subentire, pubescent on the nerves; petiole 3 to 15 mm long. Inflorescence dense cyme, 5- to many-flowered, often terminating drooping-horizontal branches, resupinate; bracts narrow; calyx 5, deeply partite, segments narrow, about 1 cm long; corollas 5, glandular-pubescent, about 3.5 cm long, dull red with green base, corolla tube long-curved, widened upwards, bilabiate, lower lip 3-lobed (turned upwards) with yellow streaks, apically sordidly yellow or greenish yellow; stamens 2, inserted in the throat, subequalling the upper lip, anther 1-celled; ovary compressed, style filiform, shortly bidentate. Fruit capsule, oblong, 4-seeded.
Description Odour, characteristic.
Macroscopical (Fig. 1a) A mixture of entire and broken, greenish brown to brown dried leaves.
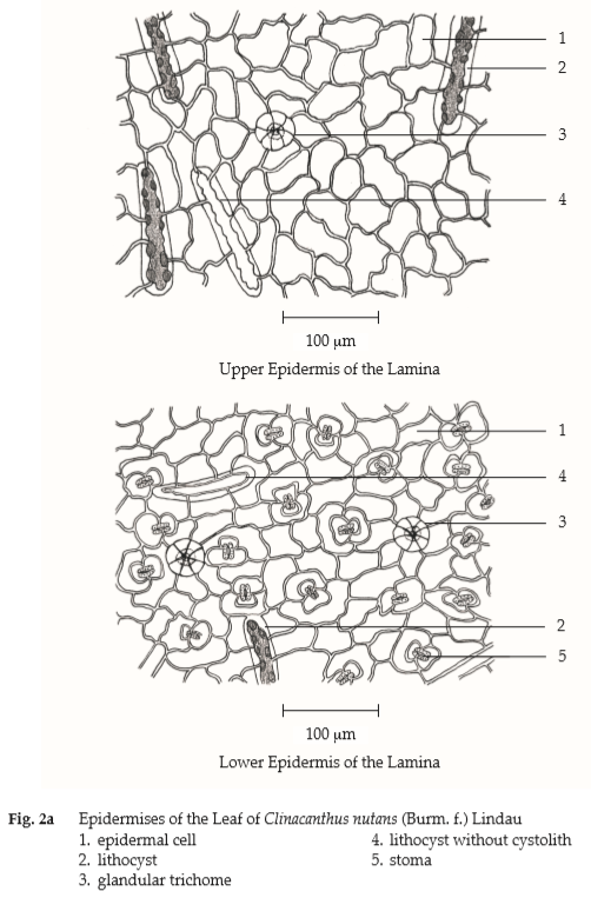
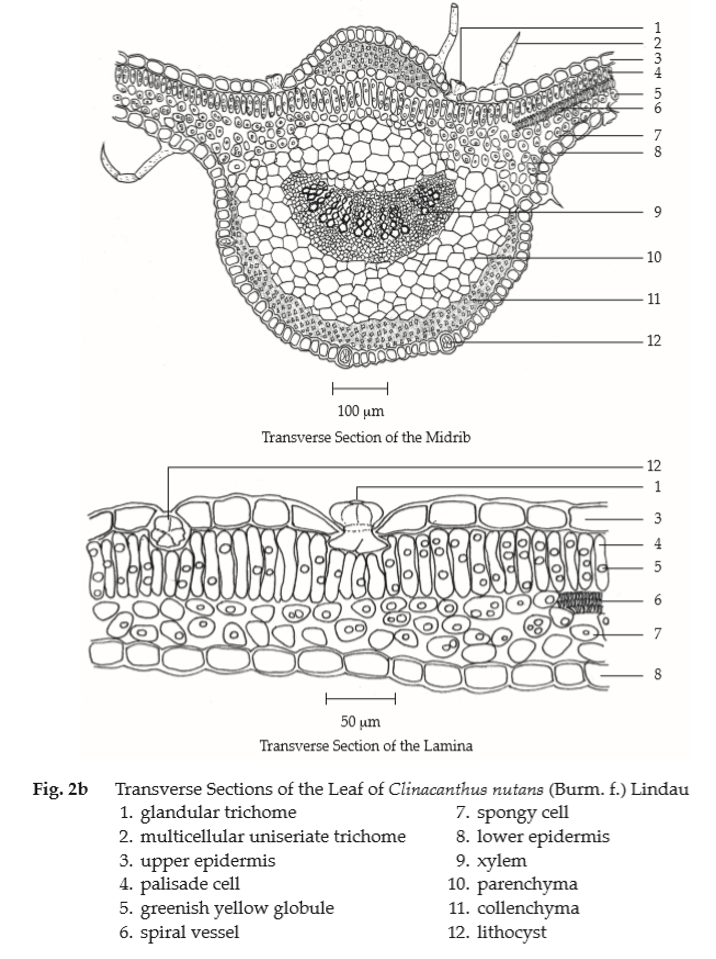
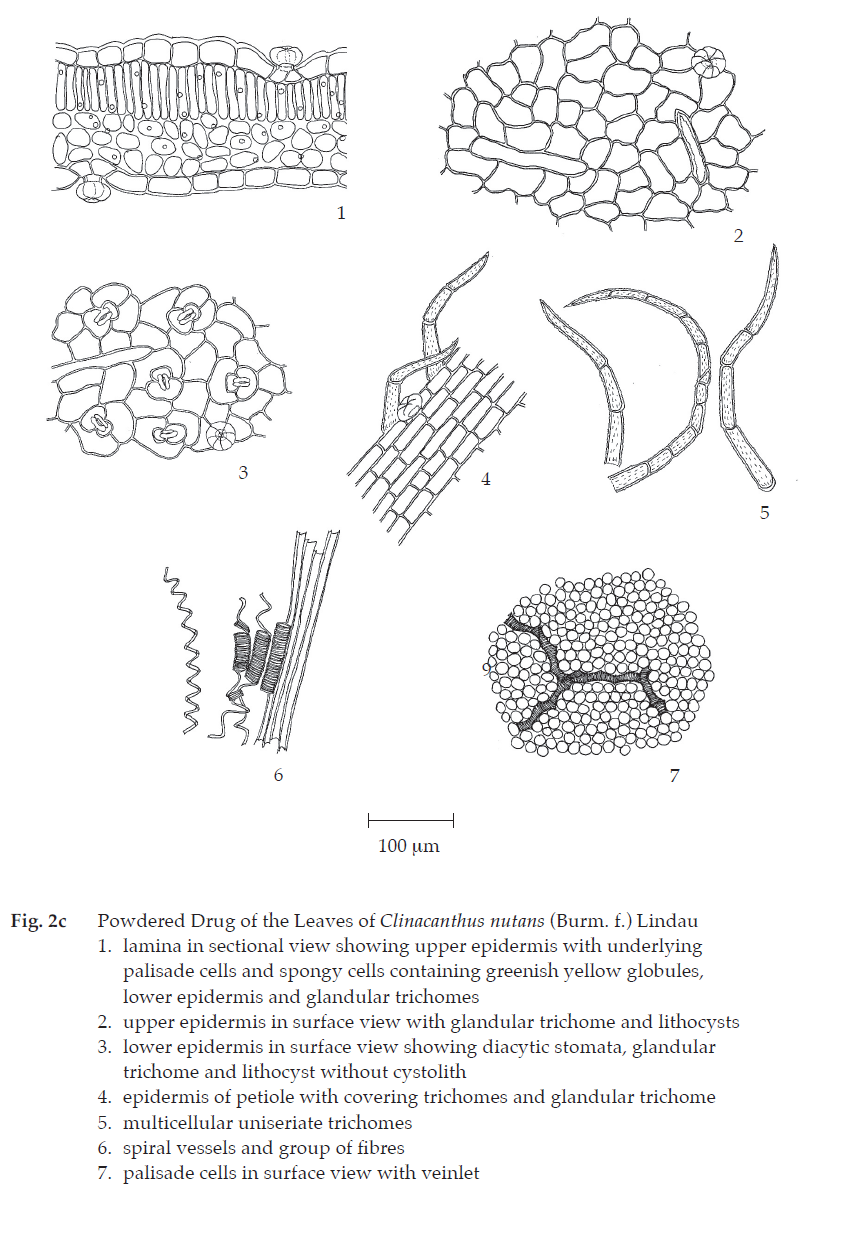
Microscopical (Figs. 2a, 2b, 2c) Transverse section of the leaf shows upper epidermis, a layer of rectangular cells, irregular and slightly wavy-walled in surface view; lithocysts, oblong; stomata absent with glandular and non-glandular trichomes. Non-glandular trichome, multicellular uniseriate, 1- to 12-celled with warted walls. Glandular trichome, unicellular stalk, 6- to 8-celled head. Lower epidermis, almost similar to those of upper epidermis, slightly wavy-walled in surface view; diacytic stomata, numerous, lithocysts and glandular trichomes. Mesophyll, a single layer of palisade cells and few layers of spongy cells, containing chloroplasts and globules of pale greenish yellow contents. Underneath both epidermises, 3 to 4 layers of angular collenchymatous cells in the midrib. Fibrovascular bundles composed of fibres, spiral vessels and xylem parenchyma.
Clinacanthus Nutans Leaf in powder possesses the diagnostic microscopical characters of the unground drug.
Warning It is not to be used on open wounds.
Additional information
1. It should be used at the early signs or symptoms of inflammation or viral infection.
2. Ethnomedically, it has been used topically as an anti-inflammatory and antiviral agents. In these cases, 10 to 15 fresh or dried leaves, crushed and soaked with sufficient amount of ethanol (40 per cent) or Thai rice whiskey are applied directly to the affected areas as often as needed. Fresh leaves are also applied directly after being crushed.

Packaging and storage Clinacanthus Nutans Leaf shall be kept in well-closed containers, protected from light, and stored in a dry place.
Identification
A. Warm 1 g of the sample, in coarse powder, with 20 mL of water in a water-bath for 10 minutes and filter (solution 1). To 2 mL of solution 1, add 1 drop of iron(III) chloride TS: a brown precipitate is produced.
B. To 2 mL of solution 1, add 1 mL of potassium cupri-tartrate TS and warm the mixture in a water-bath for a few minutes: a brick red precipitate is produced.
C. Warm 1 g of the sample, in coarse powder, with 20 mL of ethanol in a water-bath for 10 minutes and filter. Evaporate 5 mL of the filtrate to dryness. Dissolve the residue with 2 mL of acetic anhydride in a test-tube, and then slowly add 1 mL of sulfuric acid to form two layers: a greenish brown ring develops at the zone between the two layers.
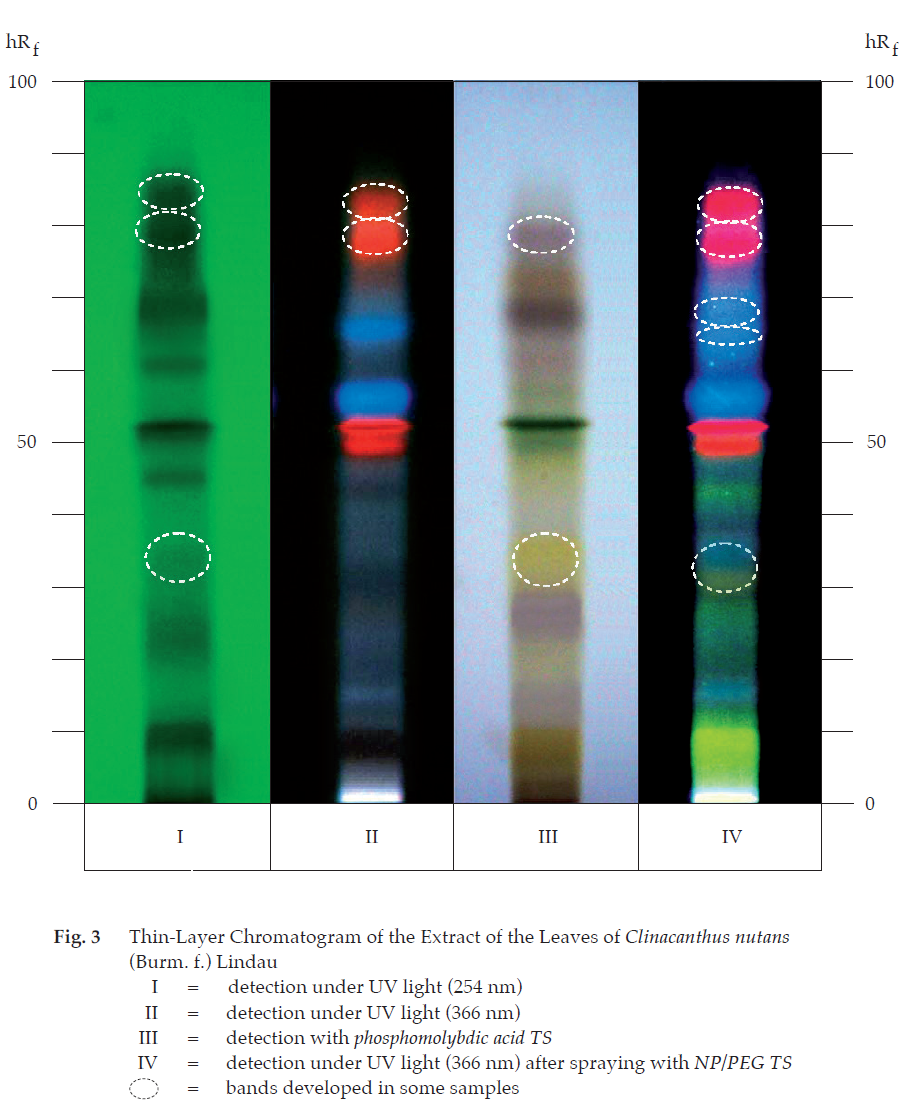
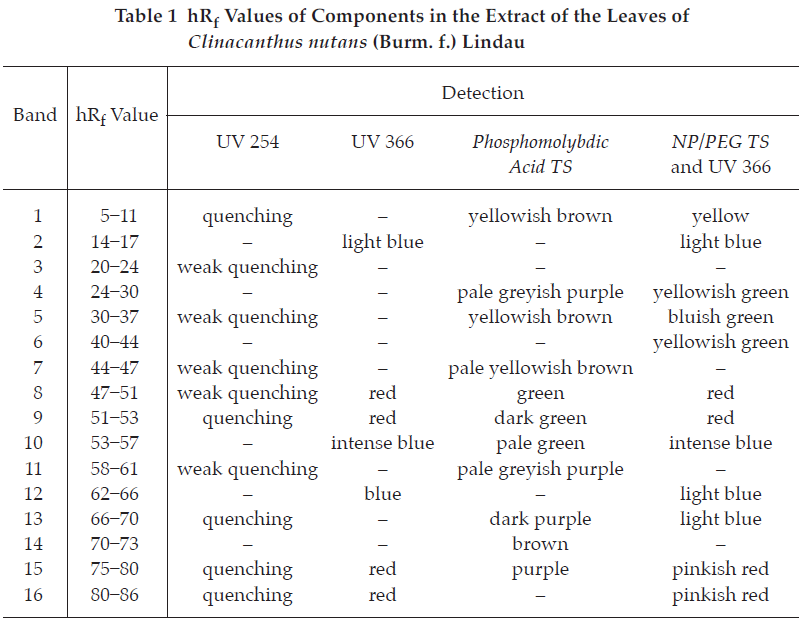
D. Carry out the test as described in the “Thin-Layer Chromatography” (Appendix 3.1), using silica gel GF254 as the coating substance and a mixture of 80 volumes of ethyl acetate and 20 volumes of a 2 per cent v/v of glacial acetic acid in methanol as the mobile phase and allowing the solvent front to ascend 12 cm above the line of application. Apply to the plate as a band of 10 mm, 10 μL of the test solution. Prepare the test solution by macerating 2 g of the sample, in coarse powder, with 30 mL of a 70 per cent v/v solution of methanol overnight and filtering. Wash 20 mL of the filtrate by shaking with three 20-mL portions of hexane, discard the washings and concentrate to 5 mL. Add 15 mL of water and extract with three 20-mL portions of ethyl acetate. Combine the ethyl acetate extracts and evaporate to dryness. Dissolve the residue in 0.5 mL of methanol. After removal of the plate, allow it to dry in air and examine under ultraviolet light (254 nm), marking the quenching bands. Several bands are observed. Examine the plate under ultraviolet light (366 nm) through the cut-off filter; several fluorescent bands of different colours are observed. Spray the plate with phosphomolybdic acid TS and heat at 105° for 5 minutes. Several bands of different colours are observed (Table 1); see also Fig. 3.
Repeat the same procedure on another plate. After removal of the plate and allow it to dry in air. Heat the plate at 80° for 10 minutes and then spray with natural products (NP) TS while the plate is still warm. Subsequently spray the plate with polyethyleneglycol (PEG) TS and observe the colours of the bands under ultraviolet light (366 nm) through the cut-off filter within 5 to 15 minutes. Several bands of different colours appear (Table 1); see also Fig. 3.
Loss on drying Not more than 12.0 per cent w/w after drying at 105° to constant weight (Appendix 4.15).
Foreign matter Not more than 2.0 per cent w/w (Appendix 7.2).
Acid-insoluble ash Not more than 1.0 per cent w/w (Appendix 7.6).
Total ash Not more than 18.0 per cent w/w (Appendix 7.7).
Ethanol (50 per cent)-soluble extractive Not less than 23.0 per cent w/w (Appendix 7.12).
Water-soluble extractive Not less than 28.0 per cent w/w (Appendix 7.12).