ตำรามาตรฐานยาสมุนไพรไทย
Thai Herbal Pharmacopoeia
สำนักยาและวัตถุเสพติด กรมวิทยาศาสตร์การแพทย์ กระทรวงสาธารณสุข
Bureau of Drug and Narcotic, Department of Medical Sciences, Ministry of Public Health(Tinospora crispa (L.) Hook.f. & Thomson)
(Nelumbo nucifera Gaertn.)
(Centella asiatica (L.) Urb.)
(Centella Dry Extract)
(Centella Cream)
(Mesua ferrea L.)
(Piper sarmentosum Roxb.)
(Piper sarmentosum Roxb.)
(Pterocarpus santalinus L. f.)
(Santalum album L.)
(Senna tora (L.) Roxb.)
(Senna alata (L.) Roxb.)
(Senna Alata Tea)
(Piper retrofractum Vahl)
(Myristica fragrans Houtt)
(Andrographis paniculata (Burm. f.) Nees)
(Andrographis Capsules)
(Allium ascalonicum L.)
(Ocimum tenuiflorum L.)
(Curcuma longa L.)
(Turmeric Capsules)
(Turmeric Dry Extract)
(Turmeric Dry Extract Capsules)
(Arcangelisia flava (L.) Merr.)
(Curcuma sp.)
Harrisonia perforata (Blanco) Merr.
(Aristolochia pierrei Lecomte)
(Zingiber officinale Roscoe)
(Ginger Capsules)
(Ginger Tea)
(Cassia fistula L.)
(Nardostachys jatamansi (D. Don) DC.)
(Angelica sinensis (Oliv.) Diels)
Artemisia annua L.
(Ligusticum sinense Oliv. cv. Chuanxiong)
(Neopicrorhiza scrophulariiflora Pennell)
(Atractylodes lancea (Thunb.) DC.)
(Aucklandia lappa Decne)
(Terminalia chebula Retz.)
(Angelica dahurica (Hoffm.) Benth. & Hook. f. ex Franch. & Sav. var. dahurica)
(Kaempferia parviflora Wall. ex Baker)
(Hibiscus sabdariffa L.)
(Roselle Tea)
(Allium sativum L.)
(Zingiber zerumbet (L.) Sm.)
(Wurfbainia testacea (Ridl.) Škorničk.& A. D. Poulsen)
(Cannabis sativa L.)
(Myristica fragrans Houtt)
(Dracaena cochinchinensis (Lour.) S. C. Chen)
(Ficus racemosa L.)
(Hyptis suaveolens (L.) Poit.)
Clerodendrum indicum (L.) Kuntze
(Phyllanthus emblica L.)
(Citrus hystrix DC.)
(Citrus hystrix DC.)
(Areca catechu L.)
(Momordica charantia L.)
Moringa oleifera Lam.
(Aegle marmelos (L.) Corrêa)
(Solanum trilobatum L.)
(Morus alba L.)
Gynostemma pentaphyllum(Thunb.)
Makino
(Clinacanthus nutans (Burm. f.) Lindau)
(Cissus quadrangularis L.)
(Mimusops elengi L.)
(Zingiber montanum (J. König) Link. ex A. Dietr.)
(Piper betle L.)
(Capsicum annuum L.)
(Capsicum Oleoresin)
(Capsicum Gel)
(Piper nigrum L.)
(Piper nigrum L.)
(Eurycoma longifolia Jack)
(Thunbergia laurifolia Lindl.)
(Piper wallichii (Miq.) Hand.-Mazz.)
Senna garrettiana (Craib) H. S. Irwin & Barneby
(Terminalia bellirica (Gaertn.) Roxb.)
(Terminalia chebula Retz.)
(Caesalpinia bonduc (L.) H. Roxb.)
(Tarlmounia elliptica (DC.) H. Rob., S. C. Keeley, Skvaria & R. Chan)
(Hog Creeper Vine Dry Extract Capsiles)
(Hog Creeper Vine Dry Extract)
(Brachypterum scandens (Roxb.) Miq.)
(Lepidium sativum L.)
(Nigella sativa L.)
(Cuminum cyminum L.)
(Foeniculum vulgare Mill.)
(Plantago ovata Forssk.)
(Pimpinella anisum L.)
(Carum carvi L.)
(Anethum graveolens L.)
(Trachyspermum ammi (L.) Sprague)
Albizia procera (Roxb.) Benth.
(Acorus calamus L.)
(Tiliacora triandra (Colebr.) Diels)
Cyanthillium cinereum (L.) H. Rob.
(Orthosiphon aristatus (Blume) Miq.)
Murdannia loriformis (Hassk.) R. S. Rao & Kammathy
(Capparis micracantha DC.)
(Chrysopogon zizanioides (L.) Roberty)
(Cyperus rotundus L.)
(Cannabis sativa L.)
(Syzygium aromaticum (L.) Merr. & L. M. Perry)
(Boesenbergia rotunda (L.) Mansf.)
(Acanthus ebracteatus Vahl)
(Acanthus ilicifolius L.)
(Kaempferia galanga L.)
(Curcuma comosa Roxb.)
Betula alnoides Buch.-Ham. ex D. Don
Cannabis sativa L.
Carthamus tinctorius L
Mitragyna speciosa (Korth.) Havil
Mallotus repandus (Rottler) Müll. Arg
Azadirachta indica A. Juss. var. siamensis Valeton
Azadirachta indica A. Juss. var. siamensis Valeton
Punica granatum L.
Rhinacanthus nasutus (L.) Kurz
Baliospermum solanifolium (Burm.) Suresh
Curcuma aeruginosa Roxb
Boesenbergia kingii Mood & L. M. Prince
Senegalia rugata (Lam.) Britton & Rose
Acacia concinna (Willd.) DC.
Senegalia rugata (Lam.) Britton & Rose
Acacia concinna (Willd.) DC.
Senna alexandriana Mill. var. alexandriana
Cassia acutifolia Delile, Cassia angustifolia Vahl
Butea superba Roxb. ex Willd.
[Plaso superba (Roxb. ex Willd.) Kuntze, Rudolphia superba (Roxb. ex Willd.) Poir.
Pueraria candollei Graham
ex Benth. var. mirifica (Airy Shaw & Suvat.) Niyomdham
Streblus asper Lour.
Suregada multiflora (A. Juss.) Baill. (Gelonium
multiflorum A. Juss.
Plumbago zeylanica L.
Plumbago indica L.
Biancaea sappan (L.) Tod.
Ziziphus attopensis Pierre
Streblus asper Lour.
Justicia gendarussa Burm. f.
Enhalus acoroides (L. f.) Royle
Bridelia ovata Decne.
Tamarindus indica L.
Citrus × aurantiifolia (Christm.) Swingle
Garcinia mangostana L.
Blumea balsamifera (L.) DC
Persicaria odorata (Lour.) Soják
Zingiber montanum (J. König) Link ex A. Dietr.
Mammea siamensis (Miq.) T. Anderson
Citrus maxima (Burm.) Merr.
Citrus × aurantium L. ‘Som Sa’
Punica granatum L.
Rhinacanthus nasutus (L.) Kurz
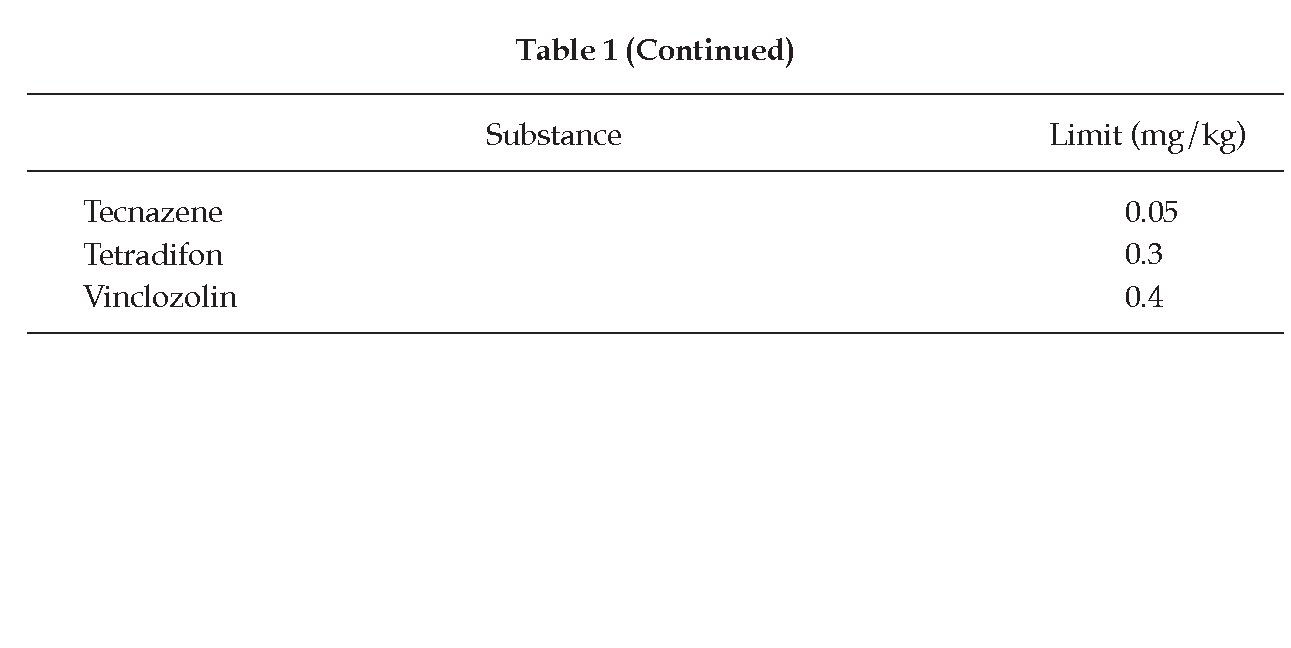
Herbal drugs are liable to contain pesticide residues which accumulate from agricultural practices, such as spraying, treatment of soils during cultivation and administration of fumigants during storage. Many herbal drug preparations of plant origin are taken over long periods of time; limits for residues should therefore be established.
Definition For the purposes of the Pharmacopoeia, a pesticide is any substance or mixture of substances intended for preventing, destroying or controlling any pest, unwanted species of plants or animals causing harm during or otherwise interfering with the production, processing, storage, transport or marketing of herbal drugs. The item includes substances intended for use as growth-regulators, defoliants or desiccants and any substance applied to crops either before or after harvest to protect the commodity from deterioration during storage and transport.
Limits Unless otherwise indicated in the monograph, the herbal drug being examined at least complies with the limits indicated in Table 1. (The calculation is based on 60 kg of body weight.) Limits for pesticides that are not listed in Table 1 are calculated using the following formula:
|
where |
ADI |
= |
acceptable daily intake, as published by FAO-WHO in milligrams per kilogram of body weight, |
|
|
M |
= |
body weight in kilograms (60 kg), and |
|
|
MDD |
= |
daily dose of the plant material in kilograms. |
If the plant herbal drug is intended for the preparation of extracts, tinctures or other pharmaceutical forms the preparation of extracts, tinctures or other pharmaceutical forms the preparation method of which modifies the content of pesticides in the finished product, the limits are calculated using the following formula:
|
where |
E |
= |
extraction factor of the method of preparation, determined experimentally. |
Higher limits can also be authorized, in exceptional cases, especially when a plant requires a particular cultivation method or has a metabolism or a structure that gives rise to a higher than normal content of pesticides.
The competent authority may grant total or partial exemption of the test when the complete history (nature and quantity of the pesticides used, date of each treatment during cultivation and after the harvest) of the treatment of the batch is known and can be checked precisely.
Sampling
METHOD For containers up to 1 kg, take one sample from the total content, thoroughly mixed, sufficuent for the test. For containers between 1 and 5 kg, take three samples, equal in volume,from the upper,middle and lower parts of the container,each being sufficient to carry out the tes ts. Thoroughly mix the samples and take from the mixture an amount sufficient to carry out the tests. For containers of more than 5 kg, take three samples, each of at least 250 g from the upper, middle and lower parts of the container. Thoroughly mix the samples and take from the mixture an amount sufficient to carry out the tests.
SIZE OF SAMPLING If the number (n) of containers is three or fewer, take samples from each container as indicated above under Method. If the number of containers is more than three,take samples as indicated under Methond,from √(n+1) containers, rounding up to the nearest unit if necessary.
The samples are to be analyzed immediately to avoid possible degradation of the residues. If this is not possible, the fresh samples should be stored in the refrigerator, but typically no longer than 5 days. Dried samples may be stored at room temperature, but if storage time is expected to exceed two weeks, they should be sub-sampled and stored in the freezer (–10°).


Qualitative and quantitative analysis of pesticide residues
Use either of the following analytical procedures:
— Analytical procedures issued by National Authorities or International Standard Organizations, or those published in internationally recognized manuals or publications;
— Analytical procedures with appropriate and reliable performance characteristics validated by a collaborative study or single-laboratory study according to the internationally recognized protocols. The validated analytical procedures shall be assessed in compliance with the latest version of ISO/IEC 17025.
The analytical procedures stated above shall be capable of providing the reliable results of the specified maximum pesticide residue level.
In addition, they shall satisfy the following criteria:
— The chosen method, especially with respect to its purification steps, is suitable for the combination of pesticide residue and substance to be examined, and is not susceptible to interference from co-extractives;
— The limit of quantification for each pesticide matrix combination to be analyzed is not more than the corresponding tolerance limit; the method is shown to recover between 70 and 120 per cent of each pesticide with a repeatability not less than 20 per cent relative standard deviation. (Note The lower recoveries may be acceptable in certain cases.)
— The concentration of the test and standard solutions and the setting of the apparatus are such that a linear response is obtained from the analytical detector.