ตำรามาตรฐานยาสมุนไพรไทย
Thai Herbal Pharmacopoeia
สำนักยาและวัตถุเสพติด กรมวิทยาศาสตร์การแพทย์ กระทรวงสาธารณสุข
Bureau of Drug and Narcotic, Department of Medical Sciences, Ministry of Public Health(Tinospora crispa (L.) Hook.f. & Thomson)
(Nelumbo nucifera Gaertn.)
(Centella asiatica (L.) Urb.)
(Centella Dry Extract)
(Centella Cream)
(Mesua ferrea L.)
(Piper sarmentosum Roxb.)
(Piper sarmentosum Roxb.)
(Pterocarpus santalinus L. f.)
(Santalum album L.)
(Senna tora (L.) Roxb.)
(Senna alata (L.) Roxb.)
(Senna Alata Tea)
(Piper retrofractum Vahl)
(Myristica fragrans Houtt)
(Andrographis paniculata (Burm. f.) Nees)
(Andrographis Capsules)
(Allium ascalonicum L.)
(Ocimum tenuiflorum L.)
(Curcuma longa L.)
(Turmeric Capsules)
(Turmeric Dry Extract)
(Turmeric Dry Extract Capsules)
(Arcangelisia flava (L.) Merr.)
(Curcuma sp.)
Harrisonia perforata (Blanco) Merr.
(Aristolochia pierrei Lecomte)
(Zingiber officinale Roscoe)
(Ginger Capsules)
(Ginger Tea)
(Cassia fistula L.)
(Nardostachys jatamansi (D. Don) DC.)
(Angelica sinensis (Oliv.) Diels)
Artemisia annua L.
(Ligusticum sinense Oliv. cv. Chuanxiong)
(Neopicrorhiza scrophulariiflora Pennell)
(Atractylodes lancea (Thunb.) DC.)
(Aucklandia lappa Decne)
(Terminalia chebula Retz.)
(Angelica dahurica (Hoffm.) Benth. & Hook. f. ex Franch. & Sav. var. dahurica)
(Kaempferia parviflora Wall. ex Baker)
(Hibiscus sabdariffa L.)
(Roselle Tea)
(Allium sativum L.)
(Zingiber zerumbet (L.) Sm.)
(Wurfbainia testacea (Ridl.) Škorničk.& A. D. Poulsen)
(Cannabis sativa L.)
(Myristica fragrans Houtt)
(Dracaena cochinchinensis (Lour.) S. C. Chen)
(Ficus racemosa L.)
(Hyptis suaveolens (L.) Poit.)
Clerodendrum indicum (L.) Kuntze
(Phyllanthus emblica L.)
(Citrus hystrix DC.)
(Citrus hystrix DC.)
(Areca catechu L.)
(Momordica charantia L.)
Moringa oleifera Lam.
(Aegle marmelos (L.) Corrêa)
(Solanum trilobatum L.)
(Morus alba L.)
Gynostemma pentaphyllum(Thunb.)
Makino
(Clinacanthus nutans (Burm. f.) Lindau)
(Cissus quadrangularis L.)
(Mimusops elengi L.)
(Zingiber montanum (J. König) Link. ex A. Dietr.)
(Piper betle L.)
(Capsicum annuum L.)
(Capsicum Oleoresin)
(Capsicum Gel)
(Piper nigrum L.)
(Piper nigrum L.)
(Eurycoma longifolia Jack)
(Thunbergia laurifolia Lindl.)
(Piper wallichii (Miq.) Hand.-Mazz.)
Senna garrettiana (Craib) H. S. Irwin & Barneby
(Terminalia bellirica (Gaertn.) Roxb.)
(Terminalia chebula Retz.)
(Caesalpinia bonduc (L.) H. Roxb.)
(Tarlmounia elliptica (DC.) H. Rob., S. C. Keeley, Skvaria & R. Chan)
(Hog Creeper Vine Dry Extract Capsiles)
(Hog Creeper Vine Dry Extract)
(Brachypterum scandens (Roxb.) Miq.)
(Lepidium sativum L.)
(Nigella sativa L.)
(Cuminum cyminum L.)
(Foeniculum vulgare Mill.)
(Plantago ovata Forssk.)
(Pimpinella anisum L.)
(Carum carvi L.)
(Anethum graveolens L.)
(Trachyspermum ammi (L.) Sprague)
Albizia procera (Roxb.) Benth.
(Acorus calamus L.)
(Tiliacora triandra (Colebr.) Diels)
Cyanthillium cinereum (L.) H. Rob.
(Orthosiphon aristatus (Blume) Miq.)
Murdannia loriformis (Hassk.) R. S. Rao & Kammathy
(Capparis micracantha DC.)
(Chrysopogon zizanioides (L.) Roberty)
(Cyperus rotundus L.)
(Cannabis sativa L.)
(Syzygium aromaticum (L.) Merr. & L. M. Perry)
(Boesenbergia rotunda (L.) Mansf.)
(Acanthus ebracteatus Vahl)
(Acanthus ilicifolius L.)
(Kaempferia galanga L.)
(Curcuma comosa Roxb.)
Betula alnoides Buch.-Ham. ex D. Don
Cannabis sativa L.
Carthamus tinctorius L
Mitragyna speciosa (Korth.) Havil
Mallotus repandus (Rottler) Müll. Arg
Azadirachta indica A. Juss. var. siamensis Valeton
Azadirachta indica A. Juss. var. siamensis Valeton
Punica granatum L.
Rhinacanthus nasutus (L.) Kurz
Baliospermum solanifolium (Burm.) Suresh
Curcuma aeruginosa Roxb
Boesenbergia kingii Mood & L. M. Prince
Senegalia rugata (Lam.) Britton & Rose
Acacia concinna (Willd.) DC.
Senegalia rugata (Lam.) Britton & Rose
Acacia concinna (Willd.) DC.
Senna alexandriana Mill. var. alexandriana
Cassia acutifolia Delile, Cassia angustifolia Vahl
Butea superba Roxb. ex Willd.
[Plaso superba (Roxb. ex Willd.) Kuntze, Rudolphia superba (Roxb. ex Willd.) Poir.
Pueraria candollei Graham
ex Benth. var. mirifica (Airy Shaw & Suvat.) Niyomdham
Streblus asper Lour.
Suregada multiflora (A. Juss.) Baill. (Gelonium
multiflorum A. Juss.
Plumbago zeylanica L.
Plumbago indica L.
Biancaea sappan (L.) Tod.
Ziziphus attopensis Pierre
Streblus asper Lour.
Justicia gendarussa Burm. f.
Enhalus acoroides (L. f.) Royle
Bridelia ovata Decne.
Tamarindus indica L.
Citrus × aurantiifolia (Christm.) Swingle
Garcinia mangostana L.
Blumea balsamifera (L.) DC
Persicaria odorata (Lour.) Soják
Zingiber montanum (J. König) Link ex A. Dietr.
Mammea siamensis (Miq.) T. Anderson
Citrus maxima (Burm.) Merr.
Citrus × aurantium L. ‘Som Sa’
Punica granatum L.
Rhinacanthus nasutus (L.) Kurz
Caraway is the dried ripe fruit of Carum carvi L. (Family Umbelliferae), Herbarium Specimen Number: see Additional information 1, Crude Drug Number: DMSc 0434.
Constituents Caraway contains volatile oil, of which (+)-carvone and (+)-limonene are its major components. It also contains fixed oils, flavonoids, etc.
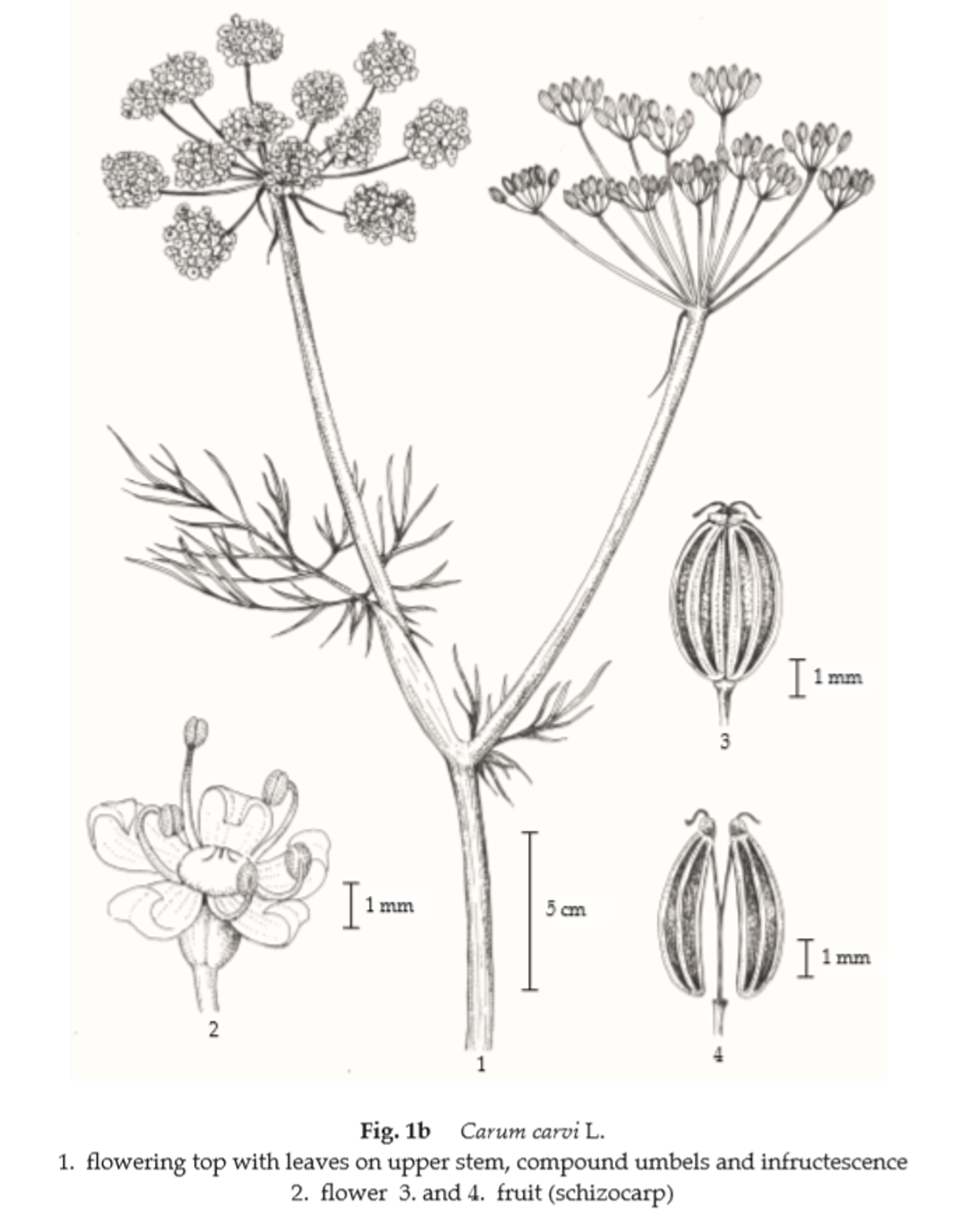
Description of the plant (Figs. 1a, 1b) Perennial herb 15 to 100 cm tall; taproot cylindrical, up to 25 cm long; stem solitary, rarely 2 to 8, base without remnant sheaths, glabrous. Leaves 2- to 3-pinnated, alternate, basal and lower leaves oblong-lanceolate in outline, 8 to 15 cm long, 3 to 8 cm wide, gradually reduced upwards, ultimate segments linear or linear- lanceolate, 3 to 5 mm long, 1 to 2 mm wide; petiole sheathing, 5 to 8 cm long. Inflorescence loose compound umbels, terminal. Flower small; pedicel extremely unequal; sepal minute or wanting; petals 5, pinkish or white, obovate with a narrower, inflexed apex; stamens 5, inserted around epigynous disk, alternating with the petals; ovary inferior, 2-loculed, ovule 1 per locule, stylopodium short conic, styles 2, short, spreading. Fruit cremocarp, brown to dark brown, oblong to ovate, 3 to 4 mm long, consisting of 2 dry, 1-seeded, indehiscent mericarps, ridges prominent, carpophore 2-fid.
Description Odour and taste, aromatic and characteristic.
Macroscopical (Fig. 1a) Cremocarp ovoid, 3 to 6 mm long, 1 to 2 mm wide; mericarp frequently separated, each being crescent shaped, slightly pubescent. Dorsal side convex, brown, with 5 longitudinal ridges and at the summit with a short conical stylopodium; commissural side concave, brown, with persisting carpophore and pedicel.
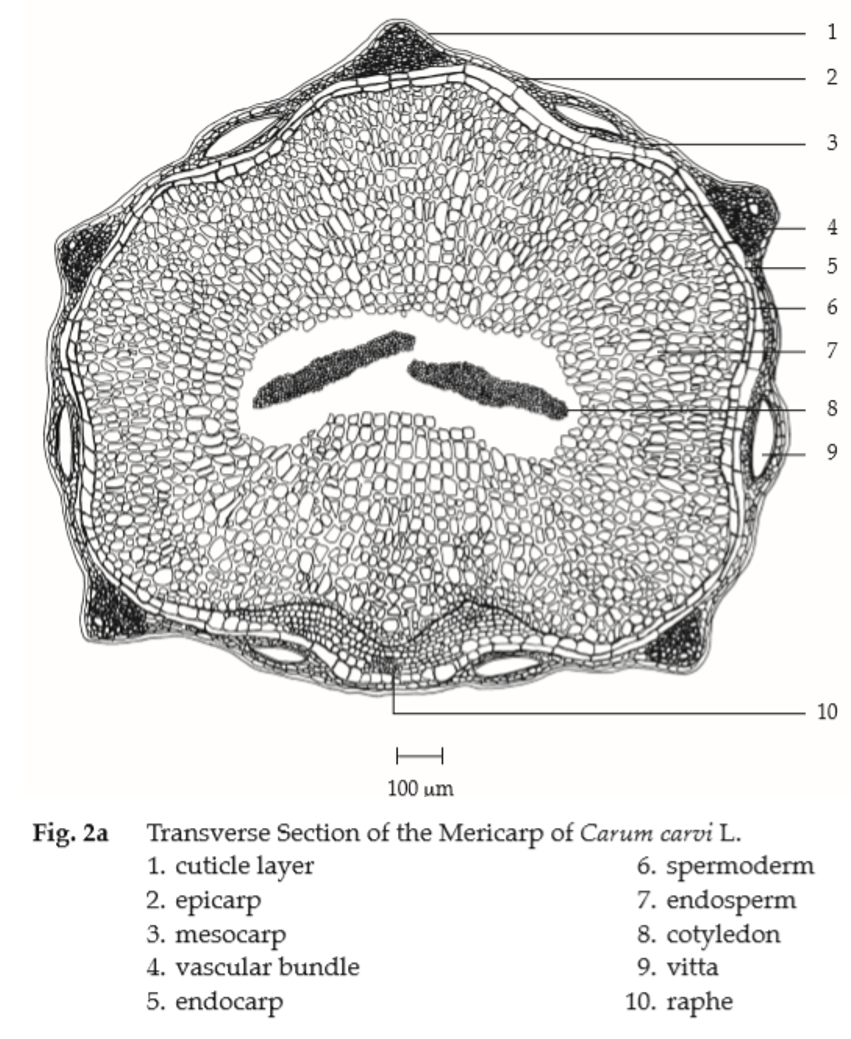
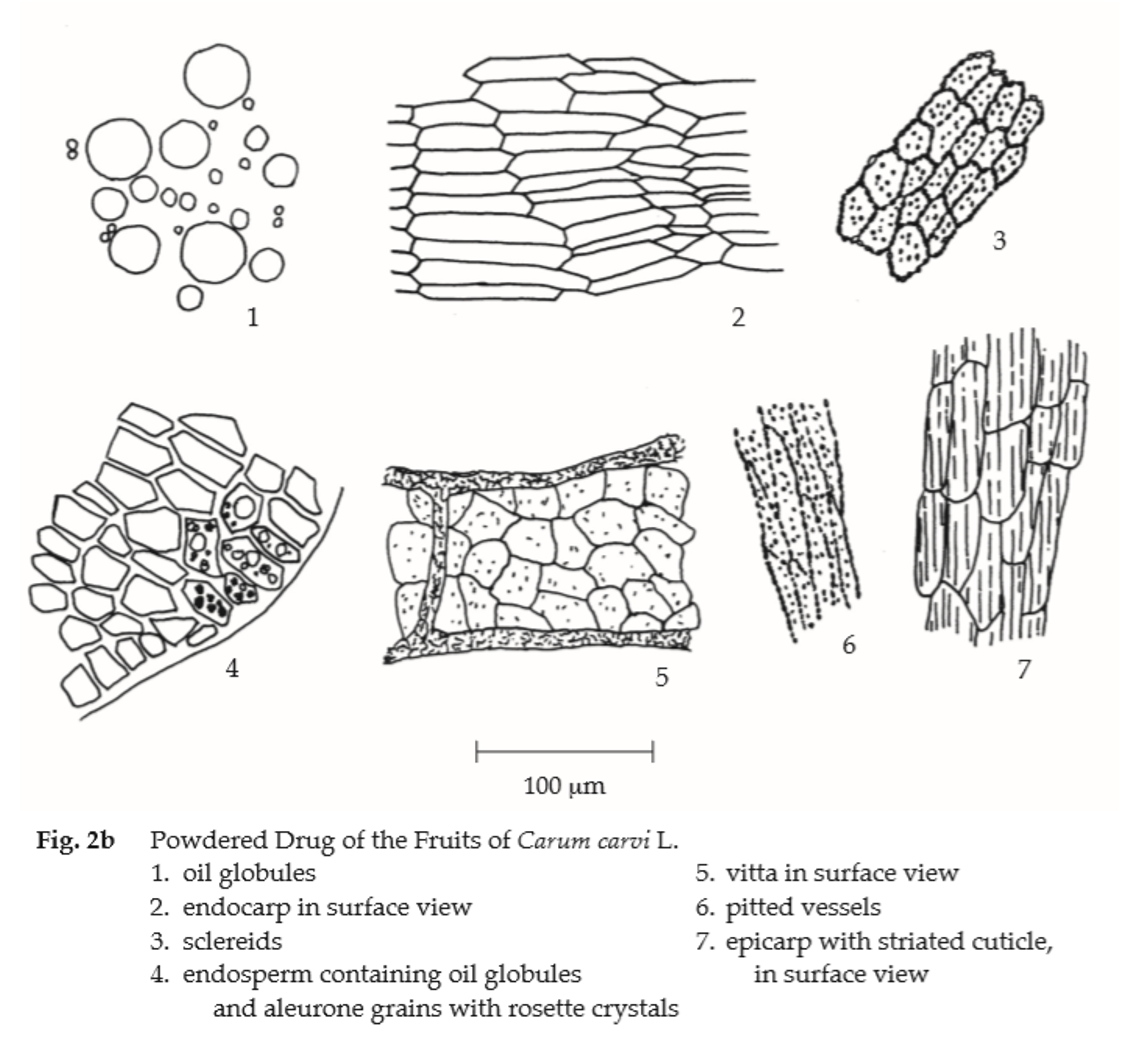
Microscopical (Figs. 2a, 2b) Transverse section of the mericarp through the cotyledon shows epicarp, mesocarp, endocarp, spermoderm, endosperm, and cotyledon. Epicarp, slightly elongated epidermal cells, covered with striated cuticle. Mesocarp, several layers of elongated parenchyma cells with more or less collapsed cells; the ridge portions, small lignified fibrovascular bundles; vittae, large, elliptic, brown, lined by small epithelial secretory cells, 4 on dorsal side between the vascular bundles and 2 on commissural side. Endocarp, a layer of broad elongated cells. Spermoderm, attached to the endocarp, consisting of a layer of brownish, elongated cells with some collapsed cells. Endosperm, thick-walled, polygonal cells containing oil globules, aleurone grains with rosette crystals. Cotyledon, small thin-walled cells containing oil globules and aleurone grains.
Caraway in powder possesses the diagnostic microscopical characters of the unground drug.

Additional information
1. Caraway plant is not native to nor commercially cultivated in Thailand. The plant yielding caraway fruit is here referred to the herbarium specimen, collector’s number J.F. Rock 14117, deposited at the Kew Herbarium (K), London, United Kingdom. The photographic illustration of the specimen can be seen at the Department of Medical Sciences Herbarium (DMSC), Nonthaburi, Thailand.
2. It is commonly used with other herbal drugs in Thai traditional herbal preparations.
Packaging and storage Caraway shall be kept in well-closed containers, preferably of metal or glass, protected from light and stored in a cool and dry place.
Identification
A. Add 5 mL of chloroform to 1 g of the sample, in powder, shake well, set aside for 30 minutes, and filter. Allow 0.1 mL of the filtrate to dry and add a few drops of a 5 per cent w/v solution of vanillin in sulfuric acid: a reddish brown colour develops and changes to red and to purple, consecutively.
B. Mix 1 g of the sample, in powder, with 3 mL of ethanol, shake for 5 minutes and filter. To 1 mL of the filtrate, add 1 mL of dinitrophenylhydrazine TS1: a turbid, orange-yellow solution is produced.
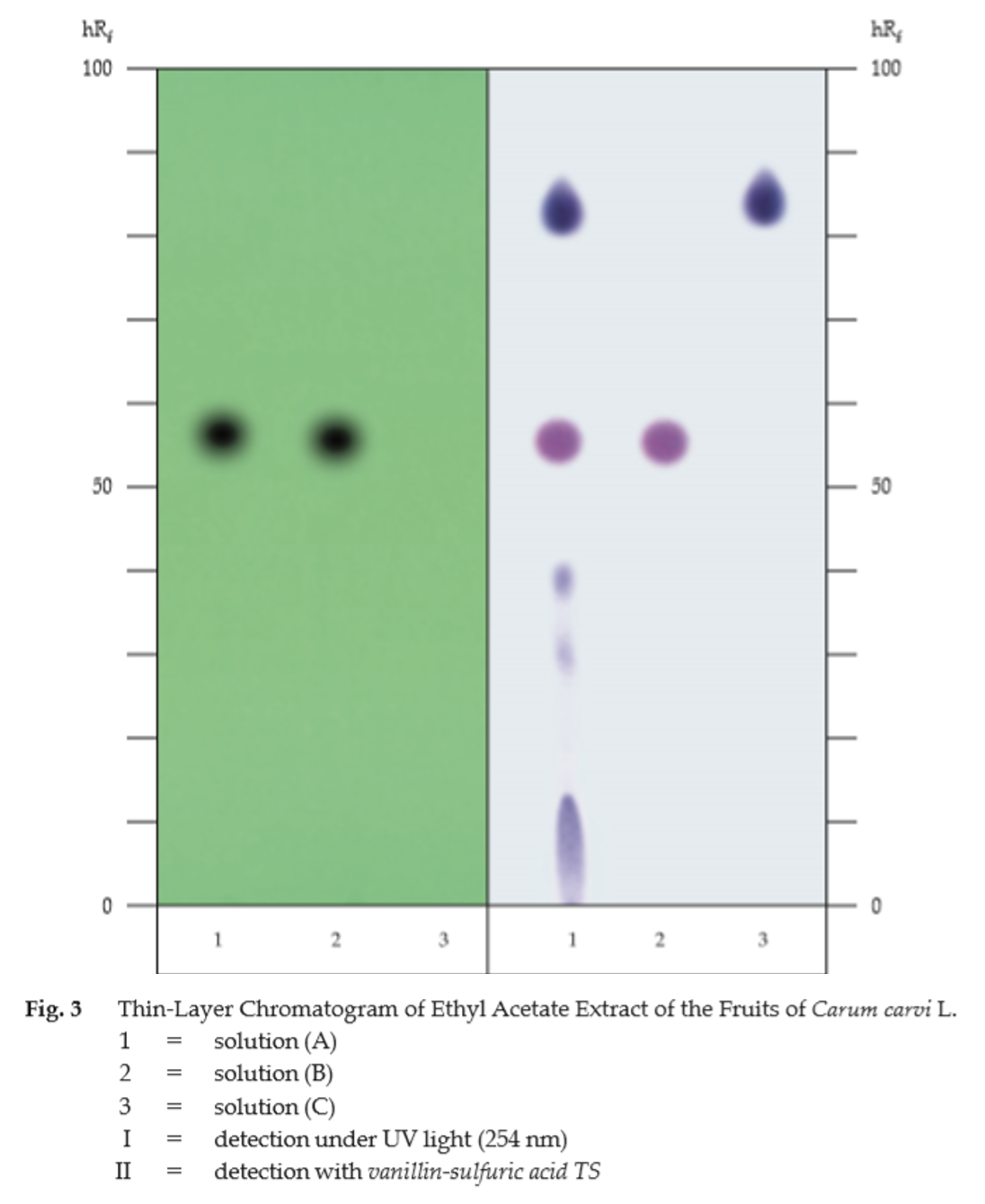
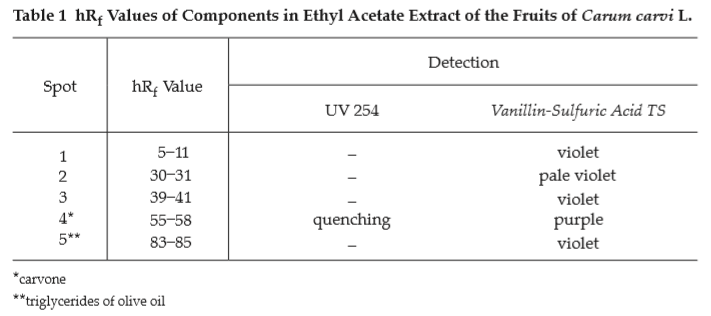
C. Carry out the test as described in the “Thin-Layer Chromatography” (Appendix 3.1), using silica gel GF254 as the coating substance and a mixture of 90 volumes of toluene and 10 volumes of ethyl acetate as the mobile phase. Apply separately to the plate, 2 μL each of solutions (A), (B) and (C). Prepare solution (A) by shaking 500 mg of sample, in fine powder, with 5 mL of ethyl acetate for 3 minutes and filtering. For solution (B), add 2 μL of carvone to 1 mL of ethyl acetate and mix. For solution (C), add 40 μL of olive oil to 1 mL of ethyl acetate and mix. After removal of the plate, allow it to dry in air and examine under ultraviolet light (254 nm), marking the quenching spots. The chromatogram obtained from solution (A) shows a quenching spot (hRf value 55 to 58) corresponding to the carvone spot from solution (B). Spray the plate with vanillin-sulfuric acid TS and heat at 110° for 10 minutes; the spot due to carvone is purple. One violet spot (hRf value 83 to 85) corresponds to the triglycerides of olive oil from solution (C). Other several spots are also observed (Table 1); see also Fig. 3.
Water Not more than 12.5 per cent v/w (Azeotropic Distillation Method, Appendix 4.12).
Foreign matter Not more than 2.0 per cent w/w (Appendix 7.2).
Acid-insoluble ash Not more than 1.5 per cent w/w (Appendix 7.6).
Total ash Not more than 7.0 per cent w/w (Appendix 7.7).
Ethanol-soluble extractive Not less than 5.5 per cent w/w (Appendix 7.12).
Volatile oil Not less than 2.5 per cent v/w, calculated on the anhydrous basis (Appendix 7.3H). Use 20 g, in coarse powder, freshly prepared and accurately weighed. Use 200 mL of water as the distillation liquid and a 500-mL round-bottomed flask. Distil at a rate of 2 to 3 mL per minute for 4 hours. Use 1.0 mL of xylene in the graduated tube.
Dose 0.5 to 2.0 g three times a day.