ตำรามาตรฐานยาสมุนไพรไทย
Thai Herbal Pharmacopoeia
สำนักยาและวัตถุเสพติด กรมวิทยาศาสตร์การแพทย์ กระทรวงสาธารณสุข
Bureau of Drug and Narcotic, Department of Medical Sciences, Ministry of Public Health(Tinospora crispa (L.) Hook.f. & Thomson)
(Nelumbo nucifera Gaertn.)
(Centella asiatica (L.) Urb.)
(Centella Dry Extract)
(Centella Cream)
(Mesua ferrea L.)
(Piper sarmentosum Roxb.)
(Piper sarmentosum Roxb.)
(Pterocarpus santalinus L. f.)
(Santalum album L.)
(Senna tora (L.) Roxb.)
(Senna alata (L.) Roxb.)
(Senna Alata Tea)
(Piper retrofractum Vahl)
(Myristica fragrans Houtt)
(Andrographis paniculata (Burm. f.) Nees)
(Andrographis Capsules)
(Allium ascalonicum L.)
(Ocimum tenuiflorum L.)
(Curcuma longa L.)
(Turmeric Capsules)
(Turmeric Dry Extract)
(Turmeric Dry Extract Capsules)
(Arcangelisia flava (L.) Merr.)
(Curcuma sp.)
Harrisonia perforata (Blanco) Merr.
(Aristolochia pierrei Lecomte)
(Zingiber officinale Roscoe)
(Ginger Capsules)
(Ginger Tea)
(Cassia fistula L.)
(Nardostachys jatamansi (D. Don) DC.)
(Angelica sinensis (Oliv.) Diels)
Artemisia annua L.
(Ligusticum sinense Oliv. cv. Chuanxiong)
(Neopicrorhiza scrophulariiflora Pennell)
(Atractylodes lancea (Thunb.) DC.)
(Aucklandia lappa Decne)
(Terminalia chebula Retz.)
(Angelica dahurica (Hoffm.) Benth. & Hook. f. ex Franch. & Sav. var. dahurica)
(Kaempferia parviflora Wall. ex Baker)
(Hibiscus sabdariffa L.)
(Roselle Tea)
(Allium sativum L.)
(Zingiber zerumbet (L.) Sm.)
(Wurfbainia testacea (Ridl.) Škorničk.& A. D. Poulsen)
(Cannabis sativa L.)
(Myristica fragrans Houtt)
(Dracaena cochinchinensis (Lour.) S. C. Chen)
(Ficus racemosa L.)
(Hyptis suaveolens (L.) Poit.)
Clerodendrum indicum (L.) Kuntze
(Phyllanthus emblica L.)
(Citrus hystrix DC.)
(Citrus hystrix DC.)
(Areca catechu L.)
(Momordica charantia L.)
Moringa oleifera Lam.
(Aegle marmelos (L.) Corrêa)
(Solanum trilobatum L.)
(Morus alba L.)
Gynostemma pentaphyllum(Thunb.)
Makino
(Clinacanthus nutans (Burm. f.) Lindau)
(Cissus quadrangularis L.)
(Mimusops elengi L.)
(Zingiber montanum (J. König) Link. ex A. Dietr.)
(Piper betle L.)
(Capsicum annuum L.)
(Capsicum Oleoresin)
(Capsicum Gel)
(Piper nigrum L.)
(Piper nigrum L.)
(Eurycoma longifolia Jack)
(Thunbergia laurifolia Lindl.)
(Piper wallichii (Miq.) Hand.-Mazz.)
Senna garrettiana (Craib) H. S. Irwin & Barneby
(Terminalia bellirica (Gaertn.) Roxb.)
(Terminalia chebula Retz.)
(Caesalpinia bonduc (L.) H. Roxb.)
(Tarlmounia elliptica (DC.) H. Rob., S. C. Keeley, Skvaria & R. Chan)
(Hog Creeper Vine Dry Extract Capsiles)
(Hog Creeper Vine Dry Extract)
(Brachypterum scandens (Roxb.) Miq.)
(Lepidium sativum L.)
(Nigella sativa L.)
(Cuminum cyminum L.)
(Foeniculum vulgare Mill.)
(Plantago ovata Forssk.)
(Pimpinella anisum L.)
(Carum carvi L.)
(Anethum graveolens L.)
(Trachyspermum ammi (L.) Sprague)
Albizia procera (Roxb.) Benth.
(Acorus calamus L.)
(Tiliacora triandra (Colebr.) Diels)
Cyanthillium cinereum (L.) H. Rob.
(Orthosiphon aristatus (Blume) Miq.)
Murdannia loriformis (Hassk.) R. S. Rao & Kammathy
(Capparis micracantha DC.)
(Chrysopogon zizanioides (L.) Roberty)
(Cyperus rotundus L.)
(Cannabis sativa L.)
(Syzygium aromaticum (L.) Merr. & L. M. Perry)
(Boesenbergia rotunda (L.) Mansf.)
(Acanthus ebracteatus Vahl)
(Acanthus ilicifolius L.)
(Kaempferia galanga L.)
(Curcuma comosa Roxb.)
Betula alnoides Buch.-Ham. ex D. Don
Cannabis sativa L.
Carthamus tinctorius L
Mitragyna speciosa (Korth.) Havil
Mallotus repandus (Rottler) Müll. Arg
Azadirachta indica A. Juss. var. siamensis Valeton
Azadirachta indica A. Juss. var. siamensis Valeton
Punica granatum L.
Rhinacanthus nasutus (L.) Kurz
Baliospermum solanifolium (Burm.) Suresh
Curcuma aeruginosa Roxb
Boesenbergia kingii Mood & L. M. Prince
Senegalia rugata (Lam.) Britton & Rose
Acacia concinna (Willd.) DC.
Senegalia rugata (Lam.) Britton & Rose
Acacia concinna (Willd.) DC.
Senna alexandriana Mill. var. alexandriana
Cassia acutifolia Delile, Cassia angustifolia Vahl
Butea superba Roxb. ex Willd.
[Plaso superba (Roxb. ex Willd.) Kuntze, Rudolphia superba (Roxb. ex Willd.) Poir.
Pueraria candollei Graham
ex Benth. var. mirifica (Airy Shaw & Suvat.) Niyomdham
Streblus asper Lour.
Suregada multiflora (A. Juss.) Baill. (Gelonium
multiflorum A. Juss.
Cumin is the dried ripe fruit of Cuminum cyminum L. (Family Umbelliferae), Crude Drug Number: DMSc 330.
Constituents Cumin contains volatile oil, of which cuminaldehyde and p-cymene are its major components. It also contains proteins, lipids, flavonoid glycosides, mucilage, etc.
Description of the plant (Fig. 1) Annual herb slender, erect, herbaceous, caulescent, glabrous, 20 to 40 cm tall. Basal leaves ovate in general outline, excluding the petioles 5 to 10 cm long, ternately dissected, the ultimate divisions linear-filiform, entire, 1 to 5 cm long; petioles sheathing, 1 to 2 cm long. Inflorescence loose compound umbels; peduncle 2 to 10 cm long; involucre of 5 to 7 linear and entire or ternate bracts with linear divisions, unequal, 1 to 5 cm long; rays 2 to 6, 5 to 25 mm long, unequal; pedicel 2 to 8 mm long. Flower small; sepal minute or wanting; petals 5, oblong with a narrower inflexed apex or emarginate, white or rose; stamens 5, alternating with the petals, inserted around an epigynous disc; ovary inferior, 2-celled, each cell 1-ovuled, styles 2, short stylopodium conic, attenuate into the rigid styles; carpophore 2-cleft to the base. Fruit mericarp, ovoid- oblong, 4 to 5 mm long, glabrous or setulose
Description Odour, aromatic; taste, spicy.
Macroscopical (Fig. 1) Entire cremocarp with pedicels attached, 4.5 to 7 mm long, 1.2 to 1.8 mm wide, oblong; mericarp, narrowly elliptical but slightly curved, covered with tiny bristly hair. Dorsal surface convex, brown, with 5 lighter-coloured filiform primary ridges, and at the summit with an acute conical stylopodium; secondary ridges occurred between each pair of primary ridges, covered with prominent bristles; commissural surface concave, brown.
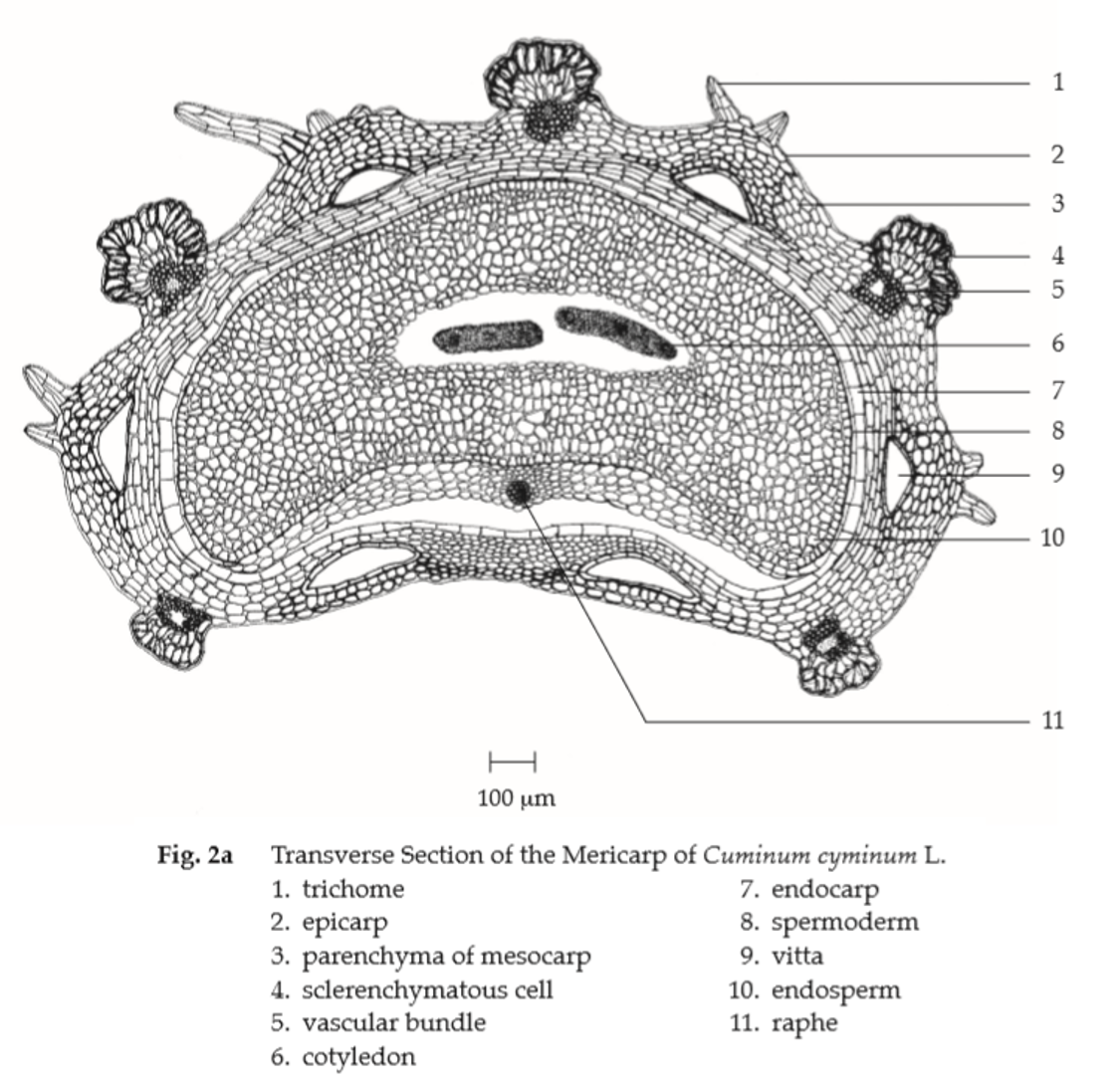
Microscopical (Figs. 2a, 2b) Transverse section of the mericarp through the cotyledon shows epicarp covered with striated cuticle; a layer of sinuous-walled epidermal cells; trichomes, multicellular, multiseriate, rounded at the apex. Mesocarp, several layers of more or less collapsed, tangentially elongated parenchyma cells; each of the ridge, sclerenchymatous cells and a lignified fibrovascular bundle; the dorsal side, 4 large vittae located between the vascular bundles; the commissural side, 2 large vittae; each vitta, elliptical, brown, lined by small epithelial secretory cells. Endocarp, a layer of tangentially elongated cells. Spermoderm, a layer of brownish, tangentially elongated cells united with the endocarp except in the region of the raphe along the commissural side where separated by collapsed thin-walled cells. Endosperm, thick-walled polygonal cells containing oil globules and aleurone grains with microcrystals. Cotyledons, thin-walled cells containing aleurone grains and oil globules.
Cumin in powder possesses the diagnostic microscopical characters of the unground drug.


Additional information It is commonly used with other herbal drugs in Thai traditional herbal preparations.
Packaging and storage Cumin shall be kept in well-closed containers, preferably of metal or glass, protected from light and stored in a cool and dry place.
Identification
A. Mix 1 g of the sample, in powder, with 3 mL of ethanol, shake for 5 minutes and filter. To 1 mL of the filtrate, add 1 mL of dinitrophenylhydrazine TS1: a turbid, orange-yellow solution is produced.
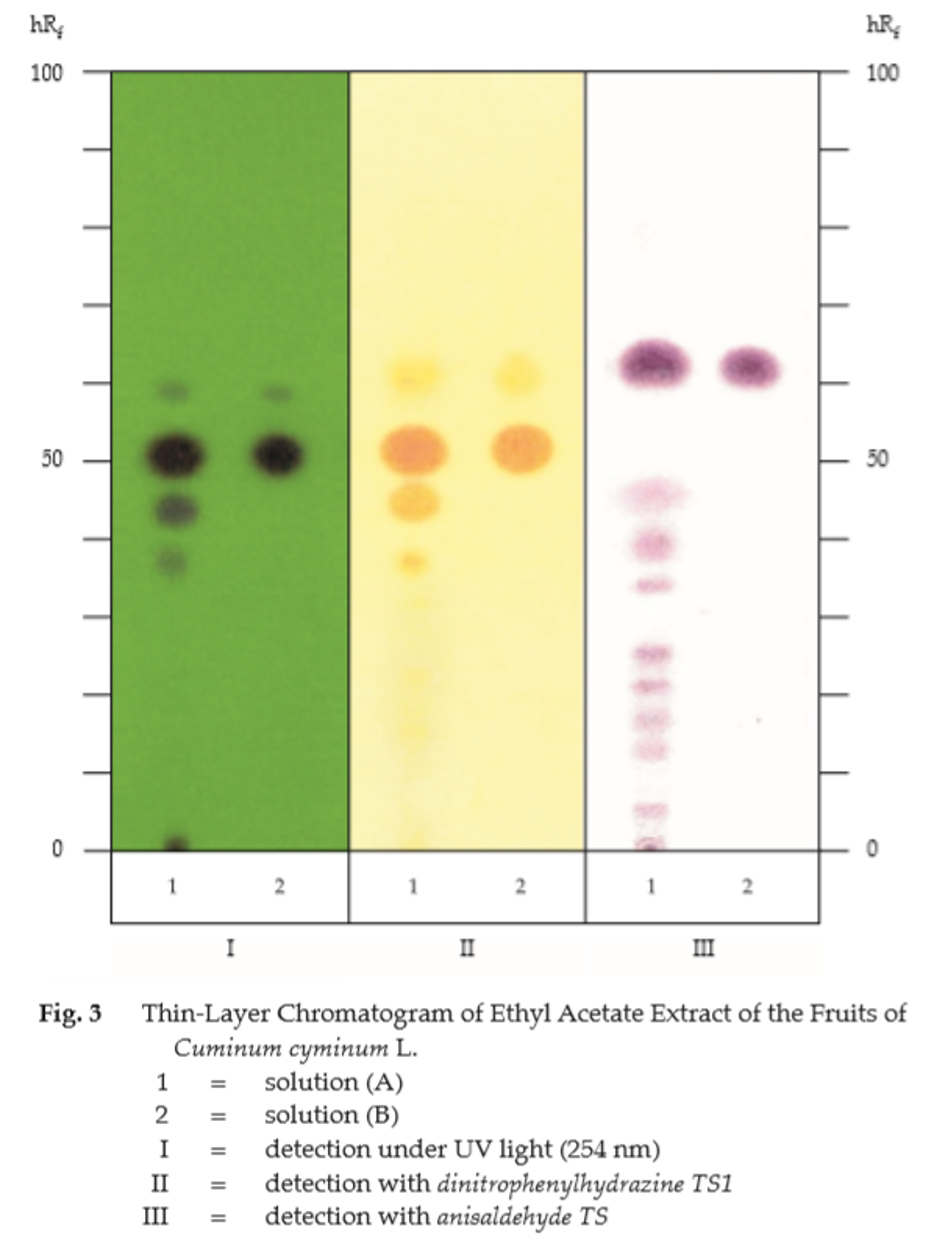
B. Carry out the test as described in the “Thin-Layer Chromatography” (Appendix 3.1), using silica gel GF254 as the coating substance and a mixture of 95 volumes of toluene and 5 volumes of ethyl acetate as the mobile phase. Apply separately to the plate, 10 μL each of solution (A) and solution (B). Prepare solution (A) by shaking 1 g of the sample, in powder, with 5 mL of ethyl acetate for 2 to 3 minutes and filtering through 2 g of anhydrous sodium sulfate. For solution (B), dissolve 2 μL of cuminaldehyde and 30 μL of olive oil in 5 mL of ethyl acetate. After removal of the plate, allow it to dry in air and examine under ultraviolet light (254 nm). The main quenching spot (hRf value 49 to 52) obtained from solution (A) corresponds to that of cuminaldehyde from solution (B). Three other quenching spots, one above and two below the main spot, are also observed. Spray the plate with dinitrophenylhydrazine TS1; the spot corresponding to cuminaldehyde appears orange while the others appear yellow (Table 1); see also Fig. 3.
Repeat the same procedure on another plate but spray with anisaldehyde TS and heat at 110° for 10 minutes. The chromatogram obtained from solution (A) shows a main violet spot (hRf value 58 to 63) corresponding to triglycerides of olive oil from solution (B); other violet spots are also observed (Table 1); see also Fig. 3.
Water Not more than 10.0 per cent v/w (Azeotropic Distillation Method, Appendix 4.12).
Foreign matter Not more than 3.0 per cent w/w of foreign matter including separated pedicels (Appendix 7.2).
Acid-insoluble ash Not more than 1.0 per cent w/w (Appendix 7.6).
Total ash Not more than 9.0 per cent w/w (Appendix 7.7).
Volatile oil Not less than 2.5 per cent v/w (Appendix 7.3H). Use 20 g, in coarse powder, freshly prepared and accurately weighed. Use 200 mL of water as the distillation liquid and a 500-mL round-bottomed flask. Distil at a rate of 2 to 3 mL per minute for 5 hours. Use 2.0 mL of xylene in the graduated tube.
Dose 0.5 to 2 g.